PKCι maintains a tumor-initiating cell phenotype that is required for ovarian tumorigenesis
- PMID: 24174471
- PMCID: PMC3888959
- DOI: 10.1158/1541-7786.MCR-13-0371-T
PKCι maintains a tumor-initiating cell phenotype that is required for ovarian tumorigenesis
Abstract
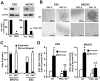
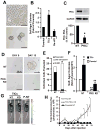
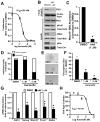
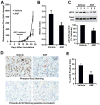
Protein kinase Cι (PKCι) has oncogenic potential and is an attractive therapeutic target for treatment of lung cancer, particularly those tumors that express elevated PKCι. However, whether PKCι is a viable target in ovarian cancer is unknown, and virtually nothing is known about the mechanism by which PKCι drives ovarian tumorigenesis. Here, it is demonstrated that PKCι maintains a tumor-initiating cell (TIC) phenotype that drives ovarian tumorigenesis. A highly tumorigenic population of cells from human ovarian cancer cell lines exhibit cancer stem-like TIC properties, including self-renewal, clonal expansion, expression of stem-related genes, enhanced transformed growth in vitro, and aggressive tumor-initiating activity in vivo. Genetic disruption of PKCι inhibits the proliferation, clonal expansion, anchorage-independent growth, and enhanced tumorigenic properties of ovarian TICs. Biochemical analysis demonstrates that PKCι acts through its oncogenic partner Ect2 to activate a MEK/ERK signaling axis that drives the ovarian TIC phenotype. Genomic analysis reveals that PKCι and Ect2 are coordinately amplified and overexpressed in the majority of primary ovarian serous tumors, and these tumors exhibit evidence of an active PKCι-Ect2 signaling axis in vivo. Finally, this study reveals that auranofin, a potent and selective inhibitor of oncogenic PKCι signaling, inhibits the tumorigenic properties of ovarian TIC cells in vitro and in vivo. These data demonstrate that PKCι is required for a TIC phenotype in ovarian cancer, and that auranofin is an attractive therapeutic option to target deadly ovarian TICs in ovarian cancer patients.
Implications: PKCι drives a tumor-initiating cell phenotype in ovarian cancer cells that can be therapeutically targeted with auranofin, a small molecule inhibitor of PKCι signaling.
Conflict of interest statement
The authors declare that they have no conflicts of interest
Figures



References
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. - PubMed
-
- Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM, et al. Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res. 2005;65:8905–11. - PubMed
-
- Takagawa R, Akimoto K, Ichikawa Y, Akiyama H, Kojima Y, Ishiguro H, et al. High expression of atypical protein kinase C lambda/iota in gastric cancer as a prognostic factor for recurrence. Ann Surg Oncol. 2010;17:81–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

