Suppression of lysosome function induces autophagy via a feedback down-regulation of MTOR complex 1 (MTORC1) activity
- PMID: 24174532
- PMCID: PMC3861628
- DOI: 10.1074/jbc.M113.511212
Suppression of lysosome function induces autophagy via a feedback down-regulation of MTOR complex 1 (MTORC1) activity
Abstract
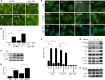
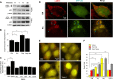
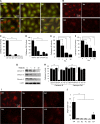
Autophagy can be activated via MTORC1 down-regulation by amino acid deprivation and by certain chemicals such as rapamycin, torin, and niclosamide. Lysosome is the degrading machine for autophagy but has also been linked to MTORC1 activation through the Rag/RRAG GTPase pathway. This association raises the question of whether lysosome can be involved in the initiation of autophagy. Toward this end, we found that niclosamide, an MTORC1 inhibitor, was able to inhibit lysosome degradation and increase lysosomal permeability. Niclosamide was ineffective in inhibiting MTORC1 in cells expressing constitutively activated Rag proteins, suggesting that its inhibitory effects were targeted to the Rag-MTORC1 signaling system. This places niclosamide in the same category of bafilomycin A1 and concanamycin A, inhibitors of the vacuolar H(+)-ATPase, for its dependence on Rag GTPase in suppression of MTORC1. Surprisingly, classical lysosome inhibitors such as chloroquine, E64D, and pepstatin A were also able to inhibit MTORC1 in a Rag-dependent manner. These lysosome inhibitors were able to activate early autophagy events represented by ATG16L1 and ATG12 puncta formation. Our work established a link between the functional status of the lysosome in general to the Rag-MTORC1 signaling axis and autophagy activation. Thus, the lysosome is not only required for autophagic degradation but also affects autophagy activation. Lysosome inhibitors can have a dual effect in suppressing autophagy degradation and in initiating autophagy.
Keywords: Autophagy; Cell Biology; Lysosomes; Signal Transduction; mTOR.
Figures


References
-
- Fleming A., Noda T., Yoshimori T., Rubinsztein D. C. (2011) Chemical modulators of autophagy as biological probes and potential therapeutics. Nat. Chem. Biol. 7, 9–17 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

