Conditional ablation of neuroprogenitor cells in adult mice impedes recovery of poststroke cognitive function and reduces synaptic connectivity in the perforant pathway
- PMID: 24174664
- PMCID: PMC3812503
- DOI: 10.1523/JNEUROSCI.2129-13.2013
Conditional ablation of neuroprogenitor cells in adult mice impedes recovery of poststroke cognitive function and reduces synaptic connectivity in the perforant pathway
Abstract
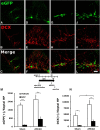
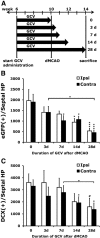
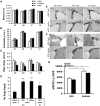
The causal relationship between neurogenesis and the recovery of poststroke cognitive function has not been properly explored. The current study aimed to determine whether depleting neuroprogenitor cells (NPCs) affects poststroke functional outcome in nestin-δ-HSV-TK-EGFP transgenic mice, in which the expression of a truncated viral thymidine kinase gene and EGFP was restricted to nestin-expressing NPCs. Ganciclovir (GCV; 200 mg/kg/d) or saline was continuously administered via osmotic pumps in mice for 4 weeks before the induction of experimental stroke. Both baseline and stroke-induced type 1 and type 2 NPCs were conditionally ablated. GCV eliminated NPCs in a duration-dependent fashion, but it did not attenuate the genesis of astroglia or oligodendrocytes in the peri-infarct cortex, nor did it affect infarct size or cerebral blood reperfusion after stroke. Transgenic stroke mice given GCV displayed impaired spatial learning and memory in the Barnes maze test compared with saline control or wild-type stroke mice given GCV, suggesting a contributing role of stroke-induced neurogenesis in the recovery of cognitive function. However, there was no significant difference in poststroke motor function between transgenic mice treated with GCV and those treated with vehicle, despite a significant ablation of NPCs in the subventricular zone of the former. Furthermore, nestin-δ-HSV-TK-EGFP mice treated with GCV had fewer retrogradely labeled neurons in the entorhinal cortex (EC) when injected with the polysynaptic viral marker PRV614 in the dentate gyrus (DG), suggesting that there might be reduced synaptic connectivity between the DG and EC following ablation of NPCs, which may contribute to impaired poststroke memory function.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
