Receptor interacting protein kinase-mediated necrosis contributes to cone and rod photoreceptor degeneration in the retina lacking interphotoreceptor retinoid-binding protein
- PMID: 24174679
- PMCID: PMC3812510
- DOI: 10.1523/JNEUROSCI.1380-13.2013
Receptor interacting protein kinase-mediated necrosis contributes to cone and rod photoreceptor degeneration in the retina lacking interphotoreceptor retinoid-binding protein
Abstract
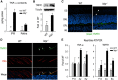
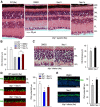
Interphotoreceptor retinoid-binding protein (IRBP) secreted by photoreceptors plays a pivotal role in photoreceptor survival with an unknown mechanism. A mutation in the human IRBP has been linked to retinitis pigmentosa, a progressive retinal degenerative disease. Mice lacking IRBP display severe early and progressive photoreceptor degeneration. However, the signaling pathway(s) leading to photoreceptor death in IRBP-deficient mice remains poorly understood. Here, we show that amounts of tumor necrosis factor-α (TNF-α) in the interphotoreceptor matrix and retinas of Irbp(-/-) mice were increased more than 10-fold and fivefold, respectively, compared with those in wild-type mice. Moreover, TNF-α receptor 1, an important membrane death receptor that mediates both programmed apoptosis and necrosis, was also significantly increased in Irbp(-/-) retina, and was colocalized with peanut agglutinin to the Irbp(-/-) cone outer segments. Although these death signaling proteins were increased, the caspase-dependent and independent apoptotic pathways were mildly activated in the Irbp(-/-) retinas, suggesting that other cell death mechanism(s) also contributes to the extensive photoreceptor degeneration in Irbp(-/-) retina. We found that receptor interacting protein 1 and 3 (RIP1 and RIP3) kinases, the intracellular key mediators of TNF-induced cellular necrosis, were elevated at least threefold in the Irbp(-/-) retinas. Moreover, pharmacological inhibition of RIP1 kinase significantly prevented cone and rod photoreceptor degeneration in Irbp(-/-) mice. These results reveal that RIP kinase-mediated necrosis strongly contributes to cone and rod degeneration in Irbp(-/-) mice, implicating the TNF-RIP pathway as a potential therapeutic target to prevent or delay photoreceptor degeneration in patients with retinitis pigmentosa caused by IRBP mutation.
Figures




Similar articles
-
Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration.Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14598-603. doi: 10.1073/pnas.1206937109. Epub 2012 Aug 20. Proc Natl Acad Sci U S A. 2012. PMID: 22908283 Free PMC article.
-
Cone outer segment and Müller microvilli pericellular matrices provide binding domains for interphotoreceptor retinoid-binding protein (IRBP).Exp Eye Res. 2013 Aug;113:192-202. doi: 10.1016/j.exer.2013.02.003. Epub 2013 Mar 5. Exp Eye Res. 2013. PMID: 23470504
-
The interphotoreceptor retinoid-binding protein (IRBP) of the chicken (Gallus gallus domesticus).Mol Vis. 2005 Sep 30;11:833-45. Mol Vis. 2005. PMID: 16254552 Free PMC article.
-
PRCD Is a Small Disc-Specific Rhodopsin-Binding Protein of Unknown Function.Adv Exp Med Biol. 2019;1185:531-535. doi: 10.1007/978-3-030-27378-1_87. Adv Exp Med Biol. 2019. PMID: 31884666 Review.
-
RIP kinase-mediated necrosis as an alternative mechanisms of photoreceptor death.Oncotarget. 2011 Jun;2(6):497-509. doi: 10.18632/oncotarget.286. Oncotarget. 2011. PMID: 21670490 Free PMC article. Review.
Cited by
-
Necrotic enlargement of cone photoreceptor cells and the release of high-mobility group box-1 in retinitis pigmentosa.Cell Death Discov. 2015 Nov 30;1:15058. doi: 10.1038/cddiscovery.2015.58. eCollection 2015. Cell Death Discov. 2015. PMID: 27551484 Free PMC article.
-
Neuroprotection for Age-Related Macular Degeneration.Ophthalmol Sci. 2022 Jul 5;2(4):100192. doi: 10.1016/j.xops.2022.100192. eCollection 2022 Dec. Ophthalmol Sci. 2022. PMID: 36570623 Free PMC article. Review.
-
Rapamycin Inhibits Light-Induced Necrosome Activation Occurring in Wild-Type, but not RPE65-Null, Mouse Retina.Invest Ophthalmol Vis Sci. 2022 Dec 1;63(13):19. doi: 10.1167/iovs.63.13.19. Invest Ophthalmol Vis Sci. 2022. PMID: 36534385 Free PMC article.
-
Identification of the zebrafish homologues of IMPG2, a retinal proteoglycan.Cell Tissue Res. 2023 Oct;394(1):93-105. doi: 10.1007/s00441-023-03808-z. Epub 2023 Jul 20. Cell Tissue Res. 2023. PMID: 37470839 Free PMC article.
-
DPP-4 Inhibitors Attenuate Fibrosis After Glaucoma Filtering Surgery by Suppressing the TGF-β/Smad Signaling Pathway.Invest Ophthalmol Vis Sci. 2023 Jul 3;64(10):2. doi: 10.1167/iovs.64.10.2. Invest Ophthalmol Vis Sci. 2023. PMID: 37405760 Free PMC article.
References
-
- Bazan NG, Reddy TS, Redmond TM, Wiggert B, Chader GJ. Endogenous fatty acids are covalently and noncovalently bound to interphotoreceptor retinoid-binding protein in the monkey retina. J Biol Chem. 1985;260:13677–13680. - PubMed
-
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. - DOI - PubMed
-
- Borst DE, Redmond TM, Elser JE, Gonda MA, Wiggert B, Chader GJ, Nickerson JM. Interphotoreceptor retinoid-binding protein. Gene characterization, protein repeat structure, and its evolution. J Biol Chem. 1989;264:1115–1123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
