Inhibitory Effect of Chrysanthemum zawadskii Herbich var. latilobum Kitamura Extract on RANKL-Induced Osteoclast Differentiation
- PMID: 24174976
- PMCID: PMC3794617
- DOI: 10.1155/2013/509482
Inhibitory Effect of Chrysanthemum zawadskii Herbich var. latilobum Kitamura Extract on RANKL-Induced Osteoclast Differentiation
Abstract
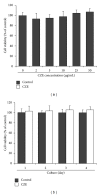
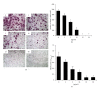
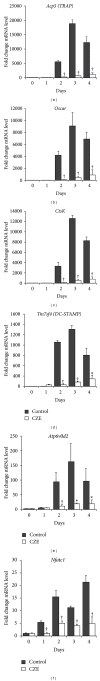
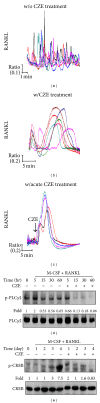
Chrysanthemum zawadskii Herbich var. latilobum Kitamura, known as "Gujulcho" in Korea, has been used in traditional medicine to treat various inflammatory diseases, including rheumatoid arthritis. However, these effects have not been tested on osteoclasts, the bone resorbing cells that regulate bone metabolism. Here, we investigated the effects of C. zawadskii Herbich var. latilobum Kitamura ethanol extract (CZE) on osteoclast differentiation induced by treatment with the receptor activator of NF- κ B ligand (RANKL). CZE inhibited osteoclast differentiation and formation in a dose-dependent manner. The inhibitory effect of CZE on osteoclastogenesis was due to the suppression of ERK activation and the ablation of RANKL-stimulated Ca(2+)-oscillation via the inactivation of PLC γ 2, followed by the inhibition of CREB activation. These inhibitory effects of CZE resulted in a significant repression of c-Fos expression and a subsequent reduction of NFATc1, a key transcription factor for osteoclast differentiation, fusion, and activation in vitro and in vivo. These results indicate that CZE negatively regulates osteoclast differentiation and may be a therapeutic candidate for the treatment of various bone diseases, such as postmenopausal osteoporosis, rheumatoid arthritis, and periodontitis.
Figures

References
-
- Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annual Review of Immunology. 2006;24:33–63. - PubMed
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. - PubMed
-
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nature Reviews Immunology. 2007;7(4):292–304. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

