Skin injuries reduce survival and modulate corticosterone, C-reactive protein, complement component 3, IgM, and prostaglandin E 2 after whole-body reactor-produced mixed field (n + γ-photons) irradiation
- PMID: 24175013
- PMCID: PMC3791621
- DOI: 10.1155/2013/821541
Skin injuries reduce survival and modulate corticosterone, C-reactive protein, complement component 3, IgM, and prostaglandin E 2 after whole-body reactor-produced mixed field (n + γ-photons) irradiation
Abstract
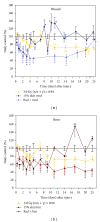
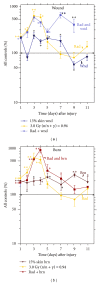
Skin injuries such as wounds or burns following whole-body γ-irradiation (radiation combined injury (RCI)) increase mortality more than whole-body γ-irradiation alone. Wound-induced decreases in survival after irradiation are triggered by sustained activation of inducible nitric oxide synthase pathways, persistent alteration of cytokine homeostasis, and increased susceptibility to systemic bacterial infection. Among these factors, radiation-induced increases in interleukin-6 (IL-6) concentrations in serum were amplified by skin wound trauma. Herein, the IL-6-induced stress proteins including C-reactive protein (CRP), complement 3 (C3), immunoglobulin M (IgM), and prostaglandin E2 (PGE2) were evaluated after skin injuries given following a mixed radiation environment that might be found after a nuclear incident. In this report, mice received 3 Gy of reactor-produced mixed field (n + γ-photons) radiations at 0.38 Gy/min followed by nonlethal skin wounding or burning. Both wounds and burns reduced survival and increased CRP, C3, and PGE2 in serum after radiation. Decreased IgM production along with an early rise in corticosterone followed by a subsequent decrease was noted for each RCI situation. These results suggest that RCI-induced alterations of corticosterone, CRP, C3, IgM, and PGE2 cause homeostatic imbalance and may contribute to reduced survival. Agents inhibiting these responses may prove to be therapeutic for RCI and improve related survival.
Figures
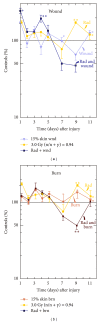
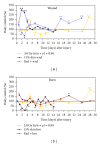
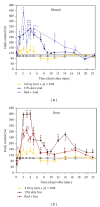
Similar articles
-
Hemorrhage enhances cytokine, complement component 3, and caspase-3, and regulates microRNAs associated with intestinal damage after whole-body gamma-irradiation in combined injury.PLoS One. 2017 Sep 21;12(9):e0184393. doi: 10.1371/journal.pone.0184393. eCollection 2017. PLoS One. 2017. PMID: 28934227 Free PMC article.
-
Wound trauma alters ionizing radiation dose assessment.Cell Biosci. 2012 Jun 11;2(1):20. doi: 10.1186/2045-3701-2-20. Cell Biosci. 2012. PMID: 22686656 Free PMC article.
-
Captopril Increases Survival after Whole-Body Ionizing Irradiation but Decreases Survival when Combined with Skin-Burn Trauma in Mice.Radiat Res. 2015 Sep;184(3):273-9. doi: 10.1667/RR14113.1. Epub 2015 Aug 25. Radiat Res. 2015. PMID: 26305295
-
Therapy of infections in mice irradiated in mixed neutron/photon fields and inflicted with wound trauma: a review of current work.Radiat Res. 1991 Oct;128(1 Suppl):S18-28. Radiat Res. 1991. PMID: 1924743 Review.
-
Tissue responses to low protracted doses of high LET radiations or photons: early and late damage relevant to radio-protective countermeasures.Adv Space Res. 1989;9(10):299-313. doi: 10.1016/0273-1177(89)90453-5. Adv Space Res. 1989. PMID: 11537307 Review.
Cited by
-
Scientific research and product development in the United States to address injuries from a radiation public health emergency.J Radiat Res. 2021 Sep 13;62(5):752-763. doi: 10.1093/jrr/rrab064. J Radiat Res. 2021. PMID: 34308479 Free PMC article.
-
Thrombopoietin Receptor Agonist Mitigates Hematopoietic Radiation Syndrome and Improves Survival after Whole-Body Ionizing Irradiation Followed by Wound Trauma.Mediators Inflamm. 2017;2017:7582079. doi: 10.1155/2017/7582079. Epub 2017 Mar 20. Mediators Inflamm. 2017. PMID: 28408792 Free PMC article.
-
Cutaneous Radiation Injuries: Models, Assessment and Treatments.Radiat Res. 2020 Sep 16;194(3):315-344. doi: 10.1667/RADE-20-00120.1. Radiat Res. 2020. PMID: 32857831 Free PMC article.
-
Circulating Cytokine/Chemokine Concentrations Respond to Ionizing Radiation Doses but not Radiation Dose Rates: Granulocyte-Colony Stimulating Factor and Interleukin-18.Radiat Res. 2018 Jun;189(6):634-643. doi: 10.1667/RR14966.1. Epub 2018 Apr 13. Radiat Res. 2018. PMID: 29652619 Free PMC article.
-
Ghrelin therapy improves survival after whole-body ionizing irradiation or combined with burn or wound: amelioration of leukocytopenia, thrombocytopenia, splenomegaly, and bone marrow injury.Oxid Med Cell Longev. 2014;2014:215858. doi: 10.1155/2014/215858. Epub 2014 Oct 13. Oxid Med Cell Longev. 2014. PMID: 25374650 Free PMC article.
References
-
- Kishi HS, Carey ME. Effects of the “special bomb”: recollections of a neurosurgeon in Hiroshima, August 8-15, 1945. Neurosurgery. 2000;47(2):441–446. - PubMed
-
- Iijima S. Pathology of atomic bomb casualties. Acta Pathologica Japonica. 1982;32(Supplement 2):237–270. - PubMed
-
- Barabanova AV. Significance of beta-radiation skin burns in Chernobyl patients for the theory and practice of radiopathology. Vojnosanitetski Pregled. 2006;63(5):477–480. - PubMed
-
- Ledney GD, Exum ED, Sheehy PA. Survival enhanced by skin-wound trauma in mice exposed to 60Co radiation. Experientia. 1981;37(2):193–194. - PubMed
-
- Ledney GD, Gelston HM, Jr., Weinberg SR, Exum ED. Survival and endogenous spleen colonies of irradiated mice after skin wounding and hydroxyurea treatment. Experientia. 1982;38(10):1228–1230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

