Sex differences in the expression of lupus-associated miRNAs in splenocytes from lupus-prone NZB/WF1 mice
- PMID: 24175965
- PMCID: PMC3843556
- DOI: 10.1186/2042-6410-4-19
Sex differences in the expression of lupus-associated miRNAs in splenocytes from lupus-prone NZB/WF1 mice
Abstract
Background: A majority of autoimmune diseases, including systemic lupus erythematosus (SLE), occur predominantly in females. Recent studies have identified specific dysregulated microRNAs (miRNAs) in both human and murine lupus, implying an important contribution of these miRNAs to lupus pathogenesis. However, to date, there is no study that examined sex differences in miRNA expression in immune cells as a plausible basis for sex differences in autoimmune disease. This study addresses this aspect in NZB/WF1 mice, a classical murine lupus model with marked female bias, and further investigates estrogen regulation of lupus-associated miRNAs.
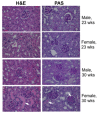
Methods: The Taqman miRNA assay system was used to quantify the miRNA expression in splenocytes from male and female NZB/WF1 mice at 17-18, 23, and 30 weeks (wks) of age. To evaluate potential estrogen's effect on lupus-associated miRNAs, 6-wk-old NZB/WF1 male mice were orchidectomized and surgically implanted with empty (placebo) or estrogen implants for 4 and 26 wks, respectively. To assess the lupus status in the NZB/WF1 mice, serum anti-dsDNA autoantibody levels, proteinuria, and renal histological changes were determined.
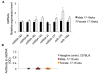
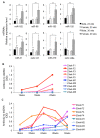
Results: The sex differences in the expression of lupus-associated miRNAs, including the miR-182-96-183 cluster, miR-155, miR-31, miR-148a, miR-127, and miR-379, were markedly evident after the onset of lupus, especially at 30 wks of age when female NZB/WF1 mice manifested moderate to severe lupus when compared to their male counterparts. Our limited data also suggested that estrogen treatment increased the expression of aforementioned lupus-associated miRNAs, with the exception of miR-155, in orchidectomized male NZB/WF1 mice to a similar level in age-matched intact female NZB/WF1 mice. It is noteworthy that orchiectomy, itself, did not affect the expression of lupus-associated miRNAs.
Conclusion: To our knowledge, this is the first study that demonstrated sex differences in the expression of lupus-associated miRNAs in splenocytes, especially in the context of autoimmunity. The increased expression of lupus-associated miRNA in female NZB/WF1 mice and conceivably in estrogen-treated orchidectomized male NZB/WF1 mice was associated with lupus manifestation. The notable increase of lupus-associated miRNAs in diseased, female NZB/WF1 mice may be a result of both lupus manifestation and the female gender.
Figures

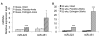
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

