Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways
- PMID: 24176089
- PMCID: PMC3978929
- DOI: 10.1186/bcr3569
Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways
Abstract
Introduction: Obesity has been associated with increased incidence and mortality of breast cancer. While the precise correlation between obesity and breast cancer remains to be determined, recent studies suggest that adipose tissue and adipose stem cells (ASCs) influence breast cancer tumorigenesis and tumor progression.
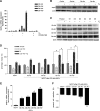
Methods: Breast cancer cells lines were co-cultured with ASCs (n = 24), categorized based on tissue site of origin and body mass index (BMI), and assessed for enhanced proliferation, alterations in gene expression profile with PCR arrays, and enhanced tumorigenesis in immunocompromised mice. The gene expression profile of ASCs was assess with PCR arrays and qRT-PCR and confirmed with Western blot analysis. Inhibitory studies were conducted by delivering estrogen antagonist ICI182,780, leptin neutralizing antibody, or aromatase inhibitor letrozole and assessing breast cancer cell proliferation. To assess the role of leptin in human breast cancers, Oncomine and Kaplan Meier plot analyses were conducted.
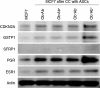
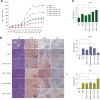
Results: ASCs derived from the abdominal subcutaneous adipose tissue of obese subjects (BMI > 30) enhanced breast cancer cell proliferation in vitro and tumorigenicity in vivo. These findings were correlated with changes in the gene expression profile of breast cancer cells after co-culturing with ASCs, particularly in estrogen receptor-alpha (ESR1) and progesterone receptor (PGR) expression. Analysis of the gene expression profile of the four groups of ASCs revealed obesity induced alterations in several key genes, including leptin (LEP). Blocking estrogen signaling with ICI182,780, leptin neutralizing antibody, or letrozole diminished the impact of ASCs derived from obese subjects. Women diagnosed with estrogen receptor/progesterone receptor positive (ER+/PR+) breast cancers that also expressed high levels of leptin had poorer prognosis than women with low leptin expression.
Conclusion: ASCs isolated from the abdomen of obese subjects demonstrated increased expression of leptin, through estrogen stimulation, which increased breast cancer cell proliferation. The results from this study demonstrate that abdominal obesity induces significant changes in the biological properties of ASCs and that these alterations enhance ER+/PR+ breast cancer tumorigenesis through estrogen dependent pathways.
Figures
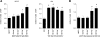


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

