TRPM7 triggers Ca2+ sparks and invadosome formation in neuroblastoma cells
- PMID: 24176224
- PMCID: PMC4912378
- DOI: 10.1016/j.ceca.2013.09.003
TRPM7 triggers Ca2+ sparks and invadosome formation in neuroblastoma cells
Abstract
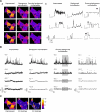
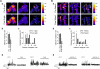
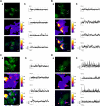
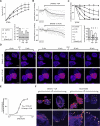
Cell migration depends on the dynamic formation and turnover of cell adhesions and is tightly controlled by actomyosin contractility and local Ca2+ signals. The divalent cation channel TRPM7 (Transient Receptor Potential cation channel, subfamily Melastatin, member 7) has recently received much attention as a regulator of cell adhesion, migration and (localized) Ca2+ signaling. Overexpression and knockdown of TRPM7 affects actomyosin contractility and the formation of cell adhesions such as invadosomes and focal adhesions, but the role of TRPM7-mediated Ca2+ signals herein is currently not understood. Using Total Internal Reflection Fluorescence (TIRF) Ca2+ fluorometry and a novel automated analysis routine we have addressed the role of Ca2+ in the control of invadosome dynamics in N1E-115 mouse neuroblastoma cells. We find that TRPM7 promotes the formation of highly repetitive and localized Ca2+ microdomains or "Ca2+ sparking hotspots" at the ventral plasma membrane. Ca2+ sparking appears strictly dependent on extracellular Ca2+ and is abolished by TRPM7 channel inhibitors such as waixenicin-A. TRPM7 inhibition also induces invadosome dissolution. However, invadosome formation is (functionally and spatially) dissociated from TRPM7-mediated Ca2+ sparks. Rather, our data indicate that TRPM7 affects actomyosin contractility and invadosome formation independent of Ca2+ influx.
Keywords: 2-APB; 2-aminoethyl diphenylborinate; Adhesion; BK; Ca(2+) imaging; Ca(2+) signaling; ECM; FA; FC; Invadosome; TIRF; TIRF microscopy; TRPM7; Total Internal Reflection Fluorescence (microscopy); Transient Receptor Potential cation channel, subfamily Melastatin, member 7; bradykinin; extracellular matrix; focal adhesion; focal complex.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int. J. Biochem. Cell Biol. 2002;34:746–761. - PubMed
-
- Rottner K, Stradal TE. Actin dynamics and turnover in cell motility. Curr. Opin. Cell Biol. 2011;23:569–578. - PubMed
-
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. - PubMed
-
- Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J. Cell Sci. 2004;117:223–231. - PubMed
-
- Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 2001;22:32–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

