Ictal high frequency oscillations distinguish two types of seizure territories in humans
- PMID: 24176977
- PMCID: PMC3859220
- DOI: 10.1093/brain/awt276
Ictal high frequency oscillations distinguish two types of seizure territories in humans
Abstract
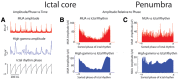
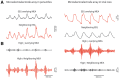
High frequency oscillations have been proposed as a clinically useful biomarker of seizure generating sites. We used a unique set of human microelectrode array recordings (four patients, 10 seizures), in which propagating seizure wavefronts could be readily identified, to investigate the basis of ictal high frequency activity at the cortical (subdural) surface. Sustained, repetitive transient increases in high gamma (80-150 Hz) amplitude, phase-locked to the low-frequency (1-25 Hz) ictal rhythm, correlated with strong multi-unit firing bursts synchronized across the core territory of the seizure. These repetitive high frequency oscillations were seen in recordings from subdural electrodes adjacent to the microelectrode array several seconds after seizure onset, following ictal wavefront passage. Conversely, microelectrode recordings demonstrating only low-level, heterogeneous neural firing correlated with a lack of high frequency oscillations in adjacent subdural recording sites, despite the presence of a strong low-frequency signature. Previously, we reported that this pattern indicates a failure of the seizure to invade the area, because of a feedforward inhibitory veto mechanism. Because multi-unit firing rate and high gamma amplitude are closely related, high frequency oscillations can be used as a surrogate marker to distinguish the core seizure territory from the surrounding penumbra. We developed an efficient measure to detect delayed-onset, sustained ictal high frequency oscillations based on cross-frequency coupling between high gamma amplitude and the low-frequency (1-25 Hz) ictal rhythm. When applied to the broader subdural recording, this measure consistently predicted the timing or failure of ictal invasion, and revealed a surprisingly small and slowly spreading seizure core surrounded by a far larger penumbral territory. Our findings thus establish an underlying neural mechanism for delayed-onset, sustained ictal high frequency oscillations, and provide a practical, efficient method for using them to identify the small ictal core regions. Our observations suggest that it may be possible to reduce substantially the extent of cortical resections in epilepsy surgery procedures without compromising seizure control.
Keywords: epilepsy surgery; high frequency oscillations; human microelectrode recordings; seizure localization.
Figures






References
-
- Alarcon G. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. Electroencephalogr Clin Neurophysiol. 1995;94:326–37. - PubMed
-
- Babb TL, Wilson CL, Isokawa-Akesson M. Firing patterns of human limbic neurons during stereoencephalography (SEEG) and clinical temporal lobe seizures. Electroencephalogr Clin Neurophysiol. 1987;66:467–482. - PubMed
-
- Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol. 2010;121:301–10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

