Cholecystokinin and pancreatic cancer: the chicken or the egg?
- PMID: 24177032
- PMCID: PMC4073990
- DOI: 10.1152/ajpgi.00301.2013
Cholecystokinin and pancreatic cancer: the chicken or the egg?
Abstract
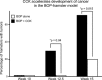
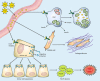
The gastrointestinal peptide cholecystokinin (CCK) causes the release of pancreatic digestive enzymes and growth of the normal pancreas. Exogenous CCK administration has been used in animal models to study pancreatitis and also as a promoter of carcinogen-induced or Kras-driven pancreatic cancer. Defining CCK receptors in normal human pancreas has been problematic because of its retroperitoneal location, high concentrations of pancreatic proteases, and endogenous RNase. Most studies indicate that the predominant receptor in human pancreas is the CCK-B type, and CCK-A is the predominant form in rodent pancreas. In pancreatic cancer cells and tumors, the role of CCK is better established because receptors are often overexpressed by these cancer cells and stimulation of such receptors promotes growth. Furthermore, in established cancer, endogenous production of CCK and/or gastrin occurs and their actions stimulate the synthesis of more receptors plus growth by an autocrine mechanism. Initially it was thought that the mechanism by which CCK served to potentiate carcinogenesis was by interplay with inflammation in the pancreatic microenvironment. But with the recent findings of CCK receptors on early PanIN (pancreatic intraepithelial neoplasia) lesions and on stellate cells, the question has been raised that perhaps CCK actions are not the result of cancer but an early driving promoter of cancer. This review will summarize what is known regarding CCK, its receptors, and pancreatic cancer, and also what is unknown and requires further investigation to determine which comes first, the chicken or the egg, "CCK or the cancer."
Keywords: G protein-coupled; cancer; cholecystokinin; gastrin; pancreas; receptor.
Figures
Similar articles
-
Cholecystokinin receptor antagonist halts progression of pancreatic cancer precursor lesions and fibrosis in mice.Pancreas. 2014 Oct;43(7):1050-9. doi: 10.1097/MPA.0000000000000194. Pancreas. 2014. PMID: 25058882 Free PMC article.
-
Differential expression of gastrin, cholecystokinin-A and cholecystokinin-B receptor mRNA in human pancreatic cancer cell lines.Scand J Gastroenterol. 2001 Jul;36(7):738-43. doi: 10.1080/003655201300192003. Scand J Gastroenterol. 2001. PMID: 11444473
-
Role of endogenous cholecystokinin on growth of human pancreatic cancer.Int J Oncol. 2011 Mar;38(3):593-601. doi: 10.3892/ijo.2010.886. Epub 2010 Dec 24. Int J Oncol. 2011. PMID: 21186400 Free PMC article.
-
[Cholecystokinin and cholecystokinin receptor].Nihon Rinsho. 1996 Apr;54(4):1097-1103. Nihon Rinsho. 1996. PMID: 8920681 Review. Japanese.
-
Gastrins, cholecystokinins and gastrointestinal cancer.Biochim Biophys Acta. 2004 Jul 6;1704(1):1-10. doi: 10.1016/j.bbcan.2004.01.004. Biochim Biophys Acta. 2004. PMID: 15238241 Review.
Cited by
-
The Benzimidazole-Based Anthelmintic Parbendazole: A Repurposed Drug Candidate That Synergizes with Gemcitabine in Pancreatic Cancer.Cancers (Basel). 2019 Dec 17;11(12):2042. doi: 10.3390/cancers11122042. Cancers (Basel). 2019. PMID: 31861153 Free PMC article.
-
Cholecystokinin Receptor Antagonist Therapy Decreases Inflammation and Fibrosis in Chronic Pancreatitis.Dig Dis Sci. 2020 May;65(5):1376-1384. doi: 10.1007/s10620-019-05863-5. Epub 2019 Oct 9. Dig Dis Sci. 2020. PMID: 31598921 Free PMC article.
-
Dietary fat stimulates pancreatic cancer growth and promotes fibrosis of the tumor microenvironment through the cholecystokinin receptor.Am J Physiol Gastrointest Liver Physiol. 2018 Nov 1;315(5):G699-G712. doi: 10.1152/ajpgi.00123.2018. Epub 2018 Jun 21. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29927319 Free PMC article.
-
Insights into Effects/Risks of Chronic Hypergastrinemia and Lifelong PPI Treatment in Man Based on Studies of Patients with Zollinger-Ellison Syndrome.Int J Mol Sci. 2019 Oct 16;20(20):5128. doi: 10.3390/ijms20205128. Int J Mol Sci. 2019. PMID: 31623145 Free PMC article. Review.
-
Cholecystokinin Receptor-Targeted Polyplex Nanoparticle Inhibits Growth and Metastasis of Pancreatic Cancer.Cell Mol Gastroenterol Hepatol. 2018 Mar 7;6(1):17-32. doi: 10.1016/j.jcmgh.2018.02.013. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 29928669 Free PMC article.
References
-
- Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC, Pal D, Briel T, Herner A, Trajkovic-Arsic M, Sipos B, Liou GY, Storz P, Murray NR, Threadgill DW, Sibilia M, Washington MK, Wilson CL, Schmid RM, Raines EW, Crawford HC, Siveke JT. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 22: 304–317, 2012 - PMC - PubMed
-
- Baldwin GS, Shulkes A. CCK receptors and cancer. Curr Top Med Chem 7: 1232–1238, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

