Tumour-on-a-chip provides an optical window into nanoparticle tissue transport
- PMID: 24177351
- PMCID: PMC3947376
- DOI: 10.1038/ncomms3718
Tumour-on-a-chip provides an optical window into nanoparticle tissue transport
Abstract
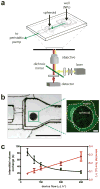
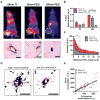
Nanomaterials are used for numerous biomedical applications, but the selection of optimal properties for maximum delivery remains challenging. Thus, there is a significant interest in elucidating the nano-bio interactions underlying tissue accumulation. To date, researchers have relied on cell culture or animal models to study nano-bio interactions. However, cell cultures lack the complexity of biological tissues and animal models are prohibitively slow and expensive. Here we report a tumour-on-a-chip system where incorporation of tumour-like spheroids into a microfluidic channel permits real-time analysis of nanoparticle (NP) accumulation at physiological flow conditions. We show that penetration of NPs into the tissue is limited by their diameter and that retention can be improved by receptor targeting. NP transport is predominantly diffusion-limited with convection improving accumulation mostly at the tissue perimeter. A murine tumour model confirms these findings and demonstrates that the tumour-on-a-chip can be useful for screening optimal NP designs prior to in vivo studies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. - PubMed
-
- Albanese A, Tang PS, Chan WCW. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annual Review of Biomedical Engineering. 2012;14:1–16. - PubMed
-
- Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

