Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis
- PMID: 24177424
- PMCID: PMC3859401
- DOI: 10.1172/JCI70338
Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis
Erratum in
- J Clin Invest. 2014 Apr 1;124(4):1868. Dosage error in article text
Abstract
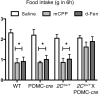
Energy and glucose homeostasis are regulated by central serotonin 2C receptors. These receptors are attractive pharmacological targets for the treatment of obesity; however, the identity of the serotonin 2C receptor-expressing neurons that mediate the effects of serotonin and serotonin 2C receptor agonists on energy and glucose homeostasis are unknown. Here, we show that mice lacking serotonin 2C receptors (Htr2c) specifically in pro-opiomelanocortin (POMC) neurons had normal body weight but developed glucoregulatory defects including hyperinsulinemia, hyperglucagonemia, hyperglycemia, and insulin resistance. Moreover, these mice did not show anorectic responses to serotonergic agents that suppress appetite and developed hyperphagia and obesity when they were fed a high-fat/high-sugar diet. A requirement of serotonin 2C receptors in POMC neurons for the maintenance of normal energy and glucose homeostasis was further demonstrated when Htr2c loss was induced in POMC neurons in adult mice using a tamoxifen-inducible POMC-cre system. These data demonstrate that serotonin 2C receptor-expressing POMC neurons are required to control energy and glucose homeostasis and implicate POMC neurons as the target for the effect of serotonin 2C receptor agonists on weight-loss induction and improved glycemic control.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

