A pathogenic non-coding RNA induces changes in dynamic DNA methylation of ribosomal RNA genes in host plants
- PMID: 24178032
- PMCID: PMC3919566
- DOI: 10.1093/nar/gkt968
A pathogenic non-coding RNA induces changes in dynamic DNA methylation of ribosomal RNA genes in host plants
Abstract
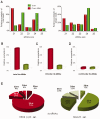
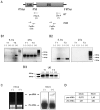
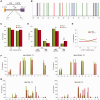
Viroids are plant-pathogenic non-coding RNAs able to interfere with as yet poorly known host-regulatory pathways and to cause alterations recognized as diseases. The way in which these RNAs coerce the host to express symptoms remains to be totally deciphered. In recent years, diverse studies have proposed a close interplay between viroid-induced pathogenesis and RNA silencing, supporting the belief that viroid-derived small RNAs mediate the post-transcriptional cleavage of endogenous mRNAs by acting as elicitors of symptoms expression. Although the evidence supporting the role of viroid-derived small RNAs in pathogenesis is robust, the possibility that this phenomenon can be a more complex process, also involving viroid-induced alterations in plant gene expression at transcriptional levels, has been considered. Here we show that plants infected with the 'Hop stunt viroid' accumulate high levels of sRNAs derived from ribosomal transcripts. This effect was correlated with an increase in the transcription of ribosomal RNA (rRNA) precursors during infection. We observed that the transcriptional reactivation of rRNA genes correlates with a modification of DNA methylation in their promoter region and revealed that some rRNA genes are demethylated and transcriptionally reactivated during infection. This study reports a previously unknown mechanism associated with viroid (or any other pathogenic RNA) infection in plants providing new insights into aspects of host alterations induced by the viroid infectious cycle.
Figures

References
-
- Ding B. The biology of viroid-host interactions. Annu. Rev. Phytopathol. 2009;47:105–131. - PubMed
-
- Ding B. Viroids: self-replicating, mobile, and fast-evolving noncoding regulatory RNAs. Wiley Interdiscip. Rev. RNA. 2010;1:362–375. - PubMed
-
- Navarro B, Gisel A, Rodio ME, Delgado S, Flores R, Di Serio F. Viroids: how to infect a host and cause disease without encoding proteins. Biochimie. 2012;94:1474–1480. - PubMed
-
- Zhao Y, Owens RA, Hammond RW. Use of a vector based on potato virus X in a whole plant assay to demonstrate nuclear targeting of potato spindle tuber viroid. J. Gen. Virol. 2001;82:1491–1497. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

