Proprotein convertase subtilisin/kexin type 7 (PCSK7) is essential for the zebrafish development and bioavailability of transforming growth factor β1a (TGFβ1a)
- PMID: 24178295
- PMCID: PMC3868773
- DOI: 10.1074/jbc.M113.453183
Proprotein convertase subtilisin/kexin type 7 (PCSK7) is essential for the zebrafish development and bioavailability of transforming growth factor β1a (TGFβ1a)
Abstract
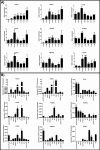
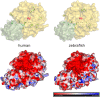
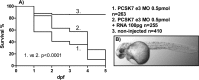
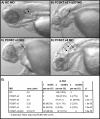
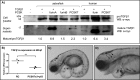
Proprotein convertase subtilisin/kexin (PCSK) enzymes convert proproteins into bioactive end products. Although other PCSK enzymes are known to be essential for biological processes ranging from cholesterol metabolism to host defense, the in vivo importance of the evolutionarily ancient PCSK7 has remained enigmatic. Here, we quantified the expressions of all pcsk genes during the 1st week of fish development and in several tissues. pcsk7 expression was ubiquitous and evident already during the early development. To compare mammalian and zebrafish PCSK7, we prepared homology models, which demonstrated remarkable structural conservation. When the PCSK7 function in developing larvae was inhibited, we found that PCSK7-deficient fish have defects in various organs, including the brain, eye, and otic vesicle, and these result in mortality within 7 days postfertilization. A genome-wide analysis of PCSK7-dependent gene expression showed that, in addition to developmental processes, several immune system-related pathways are also regulated by PCSK7. Specifically, the PCSK7 contributed to the mRNA expression and proteolytic cleavage of the cytokine TGFβ1a. Consequently, tgfβ1a morphant fish displayed phenotypical similarities with pcsk7 morphants, underscoring the importance of this cytokine in the zebrafish development. Targeting PCSK activity has emerged as a strategy for treating human diseases. Our results suggest that inhibiting PCSK7 might interfere with normal vertebrate development.
Keywords: Development; Gene Expression; Homology Modeling; Otolith; PC7; PCSK7; Proprotein Convertase; Protease; Transforming Growth Factor β (TGFβ); Zebrafish.
Figures




Similar articles
-
A genome-wide expression quantitative trait loci analysis of proprotein convertase subtilisin/kexin enzymes identifies a novel regulatory gene variant for FURIN expression and blood pressure.Hum Genet. 2015 Jun;134(6):627-36. doi: 10.1007/s00439-015-1546-5. Epub 2015 Mar 27. Hum Genet. 2015. PMID: 25813623 Clinical Trial.
-
Proprotein convertase 7 (PCSK7) reduces apoA-V levels.FEBS J. 2020 Aug;287(16):3565-3578. doi: 10.1111/febs.15212. Epub 2020 Jan 29. FEBS J. 2020. PMID: 31945259
-
cDNA structure, tissue distribution, and chromosomal localization of rat PC7, a novel mammalian proprotein convertase closest to yeast kexin-like proteinases.Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3388-93. doi: 10.1073/pnas.93.8.3388. Proc Natl Acad Sci U S A. 1996. PMID: 8622945 Free PMC article.
-
Structure and function of eukaryotic proprotein processing enzymes of the subtilisin family of serine proteases.Crit Rev Oncog. 1993;4(2):115-36. Crit Rev Oncog. 1993. PMID: 8420571 Review.
-
The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions.Biochimie. 1994;76(3-4):197-209. doi: 10.1016/0300-9084(94)90147-3. Biochimie. 1994. PMID: 7819324 Review.
Cited by
-
Myeloid cell expressed proprotein convertase FURIN attenuates inflammation.Oncotarget. 2016 Aug 23;7(34):54392-54404. doi: 10.18632/oncotarget.11106. Oncotarget. 2016. PMID: 27527873 Free PMC article.
-
Intelectin 3 is dispensable for resistance against a mycobacterial infection in zebrafish (Danio rerio).Sci Rep. 2019 Jan 30;9(1):995. doi: 10.1038/s41598-018-37678-1. Sci Rep. 2019. PMID: 30700796 Free PMC article.
-
Proprotein convertase FURIN constrains Th2 differentiation and is critical for host resistance against Toxoplasma gondii.J Immunol. 2014 Dec 1;193(11):5470-9. doi: 10.4049/jimmunol.1401629. Epub 2014 Oct 29. J Immunol. 2014. PMID: 25355923 Free PMC article.
-
PCSK7, a potential target for the treatment of age-related macular degeneration: inhibition of retinal epithelial cell death.Int J Clin Exp Pathol. 2024 Oct 15;17(10):371-380. doi: 10.62347/LEHU9944. eCollection 2024. Int J Clin Exp Pathol. 2024. PMID: 39544716 Free PMC article.
-
The motif EXEXXXL in the cytosolic tail of the secretory human proprotein convertase PC7 regulates its trafficking and cleavage activity.J Biol Chem. 2020 Feb 14;295(7):2068-2083. doi: 10.1074/jbc.RA119.011775. Epub 2020 Jan 8. J Biol Chem. 2020. PMID: 31915245 Free PMC article.
References
-
- Seidah N. G., Prat A. (2012) The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 11, 367–383 - PubMed
-
- Roebroek A. J., Umans L., Pauli I. G., Robertson E. J., van Leuven F., Van de Ven W. J., Constam D. B. (1998) Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development 125, 4863–4876 - PubMed
-
- Zhu X., Zhou A., Dey A., Norrbom C., Carroll R., Zhang C., Laurent V., Lindberg I., Ugleholdt R., Holst J. J., Steiner D. F. (2002) Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc. Natl. Acad. Sci. U.S.A. 99, 10293–10298 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

