Complexity of the alternative splicing landscape in plants
- PMID: 24179125
- PMCID: PMC3877793
- DOI: 10.1105/tpc.113.117523
Complexity of the alternative splicing landscape in plants
Abstract
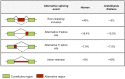
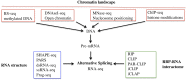
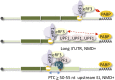
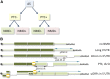
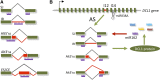
Alternative splicing (AS) of precursor mRNAs (pre-mRNAs) from multiexon genes allows organisms to increase their coding potential and regulate gene expression through multiple mechanisms. Recent transcriptome-wide analysis of AS using RNA sequencing has revealed that AS is highly pervasive in plants. Pre-mRNAs from over 60% of intron-containing genes undergo AS to produce a vast repertoire of mRNA isoforms. The functions of most splice variants are unknown. However, emerging evidence indicates that splice variants increase the functional diversity of proteins. Furthermore, AS is coupled to transcript stability and translation through nonsense-mediated decay and microRNA-mediated gene regulation. Widespread changes in AS in response to developmental cues and stresses suggest a role for regulated splicing in plant development and stress responses. Here, we review recent progress in uncovering the extent and complexity of the AS landscape in plants, its regulation, and the roles of AS in gene regulation. The prevalence of AS in plants has raised many new questions that require additional studies. New tools based on recent technological advances are allowing genome-wide analysis of RNA elements in transcripts and of chromatin modifications that regulate AS. Application of these tools in plants will provide significant new insights into AS regulation and crosstalk between AS and other layers of gene regulation.
Figures

Comment in
-
The plant cell reviews alternative splicing.Plant Cell. 2013 Oct;25(10):3639. doi: 10.1105/tpc.113.251013. Epub 2013 Oct 31. Plant Cell. 2013. PMID: 24179133 Free PMC article. No abstract available.
References
-
- Adams K.L., Wendel J.F. (2005). Polyploidy and genome evolution in plants. Curr. Opin. Plant Biol. 8: 135–141 - PubMed
-
- Ali G.S., Reddy A.S. (2008a). Spatiotemporal organization of pre-mRNA splicing proteins in plants. Curr. Top. Microbiol. Immunol. 326: 103–118 - PubMed
-
- Ali G.S., Reddy A.S. (2008b). Regulation of alternative splicing of pre-mRNAs by stresses. Curr. Top. Microbiol. Immunol. 326: 257–275 - PubMed
-
- Alló M., et al. (2009). Control of alternative splicing through siRNA-mediated transcriptional gene silencing. Nat. Struct. Mol. Biol. 16: 717–724 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

