Contrast-enhanced ultrasonography in peripheral lung consolidations: What's its actual role?
- PMID: 24179632
- PMCID: PMC3812448
- DOI: 10.4329/wjr.v5.i10.372
Contrast-enhanced ultrasonography in peripheral lung consolidations: What's its actual role?
Abstract
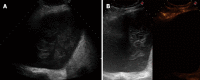
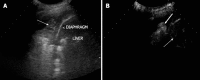
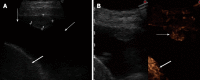
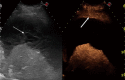
Aim: To evaluate the diagnostic accuracy of contrast-enhanced ultrasonography (CEUS) in the differential diagnosis between neoplastic and non-neoplastic peripheral pleuro-pulmonary lesions.
Methods: One hundred patients with pleural or peripheral pulmonary lesions underwent thoracic CEUS. An 8 microliters/mL solution of sulfur hexafluoride microbubbles stabilized by a phospholipid shell (SonoVue(®)) was used as US contrast agent. The clips were stored and independently reviewed by two readers, who recorded the following parameters: presence/absence of arterial enhancement, time to enhancement (TE), extent of enhancement (EE), pattern of enhancement (PE), presence/absence of wash-out, time to wash-out, and extent of wash-out. After the final diagnosis (based on histopathologic findings or follow-up of at least 15 mo) was reached, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR) of each CEUS parameter in the differential diagnosis between neoplastic and non-neoplastic lesions were calculated. Furthermore, an arbitrary score based on the ratio between the PPVs of each CEUS parameter was calculated, to evaluate if some relationship could exist between overall CEUS behaviour and neoplastic or non-neoplastic nature of the lesions.
Results: Five patients were lost at follow-up before a conclusive diagnosis was reached, 53 lesions resulted neoplastic and 42 non-neoplastic. Enhancement in the arterial phase was observed in 53/53 neoplastic lesions and 30/42 non-neoplastic lesions. On the whole, 40/42 non-neoplastic lesions showed absence of enhancement or early enhancement (95.2%) vs 3/53 neoplastic lesions (5.7%). EE was marked in 29/53 (54.7%) neoplastic lesions and 25/30 (83.3%) non-neoplastic lesions, moderate in 24/53 (45.5%) and 5/30 (16.7%), respectively. PE was homogeneous in 6/53 (11.3%) neoplastic lesions and 18/30 (60%) non-neoplastic lesions, inhomogeneous in 47/53 (88.7%) and 12/30 (40%), respectively. 19/30 (63.3%) non-neoplastic lesions enhancing in the arterial phase had no wash-out in the venous phase, 11/30 (36.7%) had late and mild wash-out. Wash-out was early in 26/53 (49%) neoplastic lesions, late in 26/53 (49%), absent in 1 (2%); marked in 16/53 (30.2%), and moderate in 36/53 (67.9%). The delayed enhancement in the arterial phase showed a sensitivity of 94.32%, specificity of 95.2%, PPV of 96.2%, NPV of 93%, PLR of 19.81, and NLR of 0.06 in identifying the neoplastic lesions. All other parameters individually considered showed unsatisfactory values of sensitivity, or specificity, or both, in differentiating neoplastic from non-neoplastic lesions. The median of the overall arbitrary score was 3 (range 0-14) in non-neoplastic lesions, and 16.5 (range 7.0-17.5) in neoplastic lesions (P < 0.001). The correlation between the diagnosis of neoplastic vs non-neoplastic lesion and the score value was statistically significant (r = 0.858, P < 0.001). Based on the score distribution, a cut-off of 7.5 enabled to reach a sensitivity of 98.1%, specificity of 95.1%, PPV 96.3%, NPV 97.5%, PVR 20.1 and NVR 0.02 in differentiating neoplastic from non-neoplastic lesions.
Conclusion: CEUS could be useful in the diagnostic workup of pleuropulmonary lesions. A delayed TE or a score ≥ 7.5 suggest the neoplastic nature of a lesion.
Keywords: Contrast-enhanced ultrasonography; Diagnostic accuracy; Neoplastic lesion; Pleuropulmonary diseases; Thoracic ultrasonography.
Figures
References
-
- Scisca C, Rizzo M, Maisano R, Monaco M, Ferrari M, Munaò S, Zavettieri M, Bonaffini O, Mare M, Rossi RT, et al. The role of ultrasound-guided aspiration biopsy of peripheral pulmonary nodules: our experience. Anticancer Res. 2002;22:2521–2523. - PubMed
-
- Tombesi P, Nielsen I, Tassinari D, Trevisani L, Abbasciano V, Sartori S. Transthoracic ultrasonography-guided core needle biopsy of pleural-based lung lesions: prospective randomized comparison between a Tru-cut-type needle and a modified Menghini-type needle. Ultraschall Med. 2009;30:390–395. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

