Estrogen replacement modulates voltage-gated potassium channels in rat presympathetic paraventricular nucleus neurons
- PMID: 24180323
- PMCID: PMC3840734
- DOI: 10.1186/1471-2202-14-134
Estrogen replacement modulates voltage-gated potassium channels in rat presympathetic paraventricular nucleus neurons
Abstract
Background: The hypothalamic paraventricular nucleus (PVN) is an important site in the regulation of the autonomic nervous system. Specifically, PVN neurons projecting to the rostral ventrolateral medulla (PVN-RVLM) play a regulatory role in the determination of the sympathetic outflow in the cardiovascular system. In the PVN-RVLM neurons, the estrogen receptor β is expressed. However, to date, the effects of estrogen on PVN-RVLM neurons have not been reported. The present study investigated estrogen-mediated modulation of two voltage-gated potassium channel (Kv) subunits, Kv4.2 and Kv4.3, that are expressed predominantly in PVN neurons and the functional current of Kv4.2 and Kv4.3, the transient outward potassium current (IA).
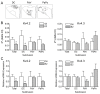
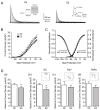
Results: Single-cell real-time RT-PCR analysis showed that 17β-estradiol (E2) replacement (once daily for 4 days) selectively down-regulated Kv4.2 mRNA levels in the PVN-RVLM neurons of ovariectomized female rats. There was no change in Kv4.3 levels. Whole-cell patch-clamp recordings demonstrated that E2 also diminished IA densities. Interestingly, these effects were most apparent in the dorsal cap parvocellular subdivision of the PVN. E2 also shortened a delay in the excitation of the PVN-RVLM neurons.
Conclusions: These findings demonstrate that E2 exerts an inhibitory effect on the functions of IA, potentially by selectively down-regulating Kv4.2 but not Kv4.3 in PVN-RVLM neurons distributed in a specific parvocellular subdivision.
Figures



References
-
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194(3):555–570. doi: 10.1002/cne.901940306. - DOI - PubMed
-
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74(2):323–364. - PubMed
-
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28(1–2):95–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

