Self-propelled micromotors for cleaning polluted water
- PMID: 24180623
- PMCID: PMC3872448
- DOI: 10.1021/nn405075d
Self-propelled micromotors for cleaning polluted water
Abstract
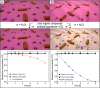
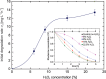
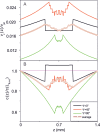
We describe the use of catalytically self-propelled microjets (dubbed micromotors) for degrading organic pollutants in water via the Fenton oxidation process. The tubular micromotors are composed of rolled-up functional nanomembranes consisting of Fe/Pt bilayers. The micromotors contain double functionality within their architecture, i.e., the inner Pt for the self-propulsion and the outer Fe for the in situ generation of ferrous ions boosting the remediation of contaminated water.The degradation of organic pollutants takes place in the presence of hydrogen peroxide, which acts as a reagent for the Fenton reaction and as main fuel to propel the micromotors. Factors influencing the efficiency of the Fenton oxidation process, including thickness of the Fe layer, pH, and concentration of hydrogen peroxide, are investigated. The ability of these catalytically self-propelled micromotors to improve intermixing in liquids results in the removal of organic pollutants ca. 12 times faster than when the Fenton oxidation process is carried out without catalytically active micromotors. The enhanced reaction-diffusion provided by micromotors has been theoretically modeled. The synergy between the internal and external functionalities of the micromotors, without the need of further functionalization, results into an enhanced degradation of nonbiodegradable and dangerous organic pollutants at small-scale environments and holds considerable promise for the remediation of contaminated water.
Figures




References
-
- Khin M. M.; Nair A. S.; Babu V. J.; Murugan R.; Ramakrishna S. A Review on Nanomaterials for Environmental Remediation. Energy Environ. Sci. 2012, 5, 8075–8109.
-
- Andreozzi R.; Caprio V.; Insola A.; Marotta R. Advanced Oxidation Processes (AOP) for Water Purification and Recovery. Catal. Today 1999, 53, 51–59.
-
- Brillas E.; Sires I.; Oturan M. A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. - PubMed
-
- Venny; Gan S. Y.; Ng N. K. Current Status and Prospects of Fenton Oxidation for the Decontamination of Persistent Organic Pollutants (POPs) in Soils. Chem. Eng. J. 2012, 213, 295–317.
-
- Hartmann M.; Kullmann S.; Keller H. Wastewater Treatment with Heterogeneous Fenton-Type Catalysts Based on Porous Materials. J. Mater. Chem. 2010, 20, 9002–9017.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

