Scaling brain size, keeping timing: evolutionary preservation of brain rhythms
- PMID: 24183025
- PMCID: PMC4009705
- DOI: 10.1016/j.neuron.2013.10.002
Scaling brain size, keeping timing: evolutionary preservation of brain rhythms
Abstract
Despite the several-thousand-fold increase of brain volume during the course of mammalian evolution, the hierarchy of brain oscillations remains remarkably preserved, allowing for multiple-time-scale communication within and across neuronal networks at approximately the same speed, irrespective of brain size. Deployment of large-diameter axons of long-range neurons could be a key factor in the preserved time management in growing brains. We discuss the consequences of such preserved network constellation in mental disease, drug discovery, and interventional therapies.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


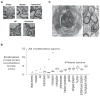
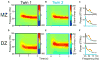
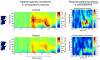
References
-
- Aboitiz F, López J, Montiel J. Long distance communication in the human brain: timing constraints for inter-hemispheric synchrony and the origin of brain lateralization. Biol Res. 2003;36:89–99. - PubMed
-
- Agid Y, Buzsáki G, Diamond DM, Frackowiak R, Giedd J, Girault JA, Grace A, Lambert JJ, Manji H, Mayberg H, et al. How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Discov. 2007;6:189–201. - PubMed
-
- Albert R, Barabási AL. Statistical mechanics of complex networks. Rev Mod Phys. 2002;74:47–97.
-
- Alhaj H, Wisniewski G, McAllister-Williams RH. The use of the EEG in measuring therapeutic drug action: focus on depression and antidepressants. J Psychopharmacol (Oxford) 2011;25:1175–1191. - PubMed
-
- Allman J. Evolving Brains. New York: Scientific American Library; 1999.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

