Luminal mitosis drives epithelial cell dispersal within the branching ureteric bud
- PMID: 24183650
- PMCID: PMC3926506
- DOI: 10.1016/j.devcel.2013.09.001
Luminal mitosis drives epithelial cell dispersal within the branching ureteric bud
Abstract
The ureteric bud is an epithelial tube that undergoes branching morphogenesis to form the renal collecting system. Although development of a normal kidney depends on proper ureteric bud morphogenesis, the cellular events underlying this process remain obscure. Here, we used time-lapse microscopy together with several genetic labeling methods to observe ureteric bud cell behaviors in developing mouse kidneys. We observed an unexpected cell behavior in the branching tips of the ureteric bud, which we term "mitosis-associated cell dispersal." Premitotic ureteric tip cells delaminate from the epithelium and divide within the lumen; although one daughter cell retains a basal process, allowing it to reinsert into the epithelium at the site of origin, the other daughter cell reinserts at a position one to three cell diameters away. Given the high rate of cell division in ureteric tips, this cellular behavior causes extensive epithelial cell rearrangements that may contribute to renal branching morphogenesis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


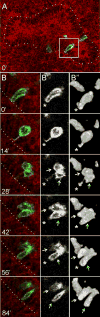
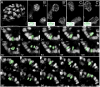
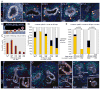


Comment in
-
Disinherited daughters travel by tube.Dev Cell. 2013 Nov 11;27(3):245-6. doi: 10.1016/j.devcel.2013.10.016. Dev Cell. 2013. PMID: 24229641
References
-
- Affolter M, Zeller R, Caussinus E. Tissue remodelling through branching morphogenesis. Nat Rev Mol Cell Biol. 2009;10:831–842. - PubMed
-
- Al-Awqati Q, Goldberg MR. Architectural patterns in branching morphogenesis in the kidney. Kidney International. 1998;54:1832–1842. - PubMed
-
- Baena-Lopez LA, Baonza A, Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol. 2005;15:1640–1644. - PubMed
-
- Baker J, Garrod D. Epithelial cells retain junctions during mitosis. J Cell Sci. 1993;104(Pt 2):415–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

