Platelet-derived growth factor receptor alpha (PDGFRα) targeting and relevant biomarkers in ovarian carcinoma
- PMID: 24183729
- PMCID: PMC3946949
- DOI: 10.1016/j.ygyno.2013.10.027
Platelet-derived growth factor receptor alpha (PDGFRα) targeting and relevant biomarkers in ovarian carcinoma
Abstract
Objective: Platelet-derived growth factor receptor alpha (PDGFRα) is believed to be associated with cell survival. We examined (i) whether PDGFRα blockade enhances the antitumor activity of taxanes in ovarian carcinoma and (ii) potential biomarkers of response to anti-PDGFRα therapy.
Methods: PDGFRα expression in 176 ovarian carcinomas was evaluated with tissue microarray and correlated to survival outcome. Human-specific monoclonal antibody to PDGFRα (IMC-3G3) was used for in vitro and in vivo experiments with or without docetaxel. Gene microarrays and reverse-phase protein arrays with pathway analyses were performed to identify potential predictive biomarkers.
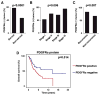
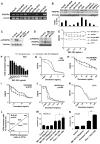
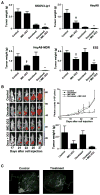
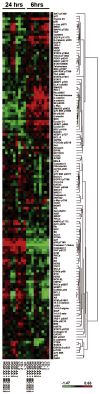
Results: When compared to low or no PDGFRα expression, increased PDGFRα expression was associated with significantly poorer overall survival of patients with ovarian cancer (P=0.014). Although treatment with IMC-3G3 alone did not affect cell viability or increase apoptosis, concurrent use of IMC-3G3 with docetaxel significantly enhanced sensitization to docetaxel and apoptosis. In an orthotopic mouse model, IMC-3G3 monotherapy had no significant antitumor effects in SKOV3-ip1 (low PDGFRα expression), but showed significant antitumor effects in HeyA8-MDR (high PDGFRα expression). Concurrent use of IMC-3G3 with docetaxel, compared with use of docetaxel alone, significantly reduced tumor weight in all tested cell lines. In protein ontology, the EGFR and AKT pathways were downregulated by IMC-3G3 therapy. MAPK and CCNB1 were downregulated only in the HeyA8-MDR model.
Conclusion: These data identify IMC-3G3 as an attractive therapeutic strategy and identify potential predictive markers for further development.
Keywords: EGFR; IMC-3G3; MAPK; Ovarian cancer; Platelet-derived growth factor receptor alpha.
© 2013.
Conflict of interest statement
Dr. Loizos is employed by ImClone Systems, a wholly owned subsidiary of Eli Lilly. The other authors declare that there is no conflict of interest in the study.
Figures

Similar articles
-
Biologic effects of platelet-derived growth factor receptor α blockade in uterine cancer.Clin Cancer Res. 2014 May 15;20(10):2740-50. doi: 10.1158/1078-0432.CCR-13-2507. Epub 2014 Mar 14. Clin Cancer Res. 2014. PMID: 24634380 Free PMC article.
-
Targeting the {alpha} receptor for platelet-derived growth factor as a primary or combination therapy in a preclinical model of prostate cancer skeletal metastasis.Clin Cancer Res. 2010 Oct 15;16(20):5002-10. doi: 10.1158/1078-0432.CCR-10-1863. Epub 2010 Sep 2. Clin Cancer Res. 2010. PMID: 20813817
-
Stromal platelet-derived growth factor receptor α (PDGFRα) provides a therapeutic target independent of tumor cell PDGFRα expression in lung cancer xenografts.Mol Cancer Ther. 2012 Nov;11(11):2473-82. doi: 10.1158/1535-7163.MCT-12-0431. Epub 2012 Aug 28. Mol Cancer Ther. 2012. PMID: 22933705 Free PMC article.
-
Olaratumab in the management of advanced soft tissue sarcoma.J Oncol Pharm Pract. 2019 Mar;25(2):442-448. doi: 10.1177/1078155218788135. Epub 2018 Jul 22. J Oncol Pharm Pract. 2019. PMID: 30032714 Review.
-
IMC-C225, an anti-epidermal growth factor receptor monoclonal antibody for treatment of head and neck cancer.Semin Oncol. 2002 Oct;29(5 Suppl 14):18-30. doi: 10.1053/sonc.2002.35644. Semin Oncol. 2002. PMID: 12422310 Review.
Cited by
-
Platelet-derived growth factor (PDGF) signalling in cancer: rapidly emerging signalling landscape.Cell Biochem Funct. 2015 Jul;33(5):257-65. doi: 10.1002/cbf.3120. Epub 2015 Jul 7. Cell Biochem Funct. 2015. PMID: 26153649 Free PMC article. Review.
-
Dihydroartemisinin selectively inhibits PDGFRα-positive ovarian cancer growth and metastasis through inducing degradation of PDGFRα protein.Cell Discov. 2017 Nov 21;3:17042. doi: 10.1038/celldisc.2017.42. eCollection 2017. Cell Discov. 2017. PMID: 29387451 Free PMC article.
-
Randomized phase II study of the PDGFRα antibody olaratumab plus liposomal doxorubicin versus liposomal doxorubicin alone in patients with platinum-refractory or platinum-resistant advanced ovarian cancer.BMC Cancer. 2018 Dec 27;18(1):1292. doi: 10.1186/s12885-018-5198-4. BMC Cancer. 2018. PMID: 30591028 Free PMC article. Clinical Trial.
-
Anlotinib combined with etoposide for platinum-resistant recurrent ovarian cancer: A case report.Medicine (Baltimore). 2020 May;99(20):e20053. doi: 10.1097/MD.0000000000020053. Medicine (Baltimore). 2020. PMID: 32443311 Free PMC article.
-
Therapeutic targets and new directions for antibodies developed for ovarian cancer.MAbs. 2016 Nov/Dec;8(8):1437-1455. doi: 10.1080/19420862.2016.1219005. Epub 2016 Aug 5. MAbs. 2016. PMID: 27494775 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
-
- Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian and peritoneal cancer. Int J Cancer. 2009;125(11):2721–7. - PubMed
-
- Oseini AM, Roberts LR. PDGFRalpha: a new therapeutic target in the treatment of hepatocellular carcinoma? Expert Opin Ther Targets. 2009;13(4):443–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

