Mucosal pre-exposure to Th17-inducing adjuvants exacerbates pathology after influenza infection
- PMID: 24183780
- PMCID: PMC3873493
- DOI: 10.1016/j.ajpath.2013.09.012
Mucosal pre-exposure to Th17-inducing adjuvants exacerbates pathology after influenza infection
Abstract
Mucosal vaccines are thought to confer superior protection against mucosal infectious diseases. In addition, mucosal routes of vaccine delivery preferentially induce the generation of T helper 17 (Th17) cells, which produce the cytokine IL-17. Th17 cells are critical in mediating vaccine-induced immunity against several mucosal infectious diseases. However, IL-17 is also a potent proinflammatory cytokine, and we recently showed that IL-17 mediates immunopathology and lung injury after influenza infection in mice. In the present study, we tested the hypothesis that mucosal pre-exposure to Th17-inducing adjuvants can promote disease exacerbation upon subsequent infection with influenza virus. Mice mucosally pre-exposed to Th17-inducing adjuvants, such as type II heat-labile enterotoxin or cholera toxin, resulted in increased morbidity and exacerbated lung inflammation upon subsequent infection with influenza virus. Furthermore, the increased morbidity was accompanied by increased expression of inflammatory chemokines and increased accumulation of neutrophils. Importantly, blockade of the IL-17 pathway in mice pre-exposed to Th17-inducing adjuvants resulted in attenuation of the inflammatory phenotype seen in influenza-infected mice. Our findings indicate that, before mucosal Th17-inducing adjuvants can be used in vaccine strategies, the short- and long-term detrimental effects of such adjuvants on disease exacerbation and lung injury in response to infections, such as influenza, should be carefully studied.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
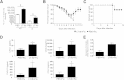
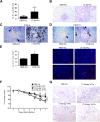
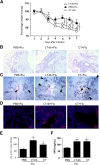
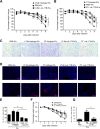
Similar articles
-
Mechanisms of Cholera Toxin in the Modulation of TH17 Responses.Crit Rev Immunol. 2015;35(2):135-52. doi: 10.1615/critrevimmunol.2015012295. Crit Rev Immunol. 2015. PMID: 26351147 Free PMC article. Review.
-
Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus.J Virol. 2010 Dec;84(24):12703-12. doi: 10.1128/JVI.01182-10. Epub 2010 Sep 29. J Virol. 2010. PMID: 20881038 Free PMC article.
-
Sublingual immunization with a subunit influenza vaccine elicits comparable systemic immune response as intramuscular immunization, but also induces local IgA and TH17 responses.Vaccine. 2014 Apr 25;32(20):2382-8. doi: 10.1016/j.vaccine.2013.12.043. Epub 2014 Jan 13. Vaccine. 2014. PMID: 24434044
-
Rationalized design of a mucosal vaccine protects against Mycobacterium tuberculosis challenge in mice.J Leukoc Biol. 2017 Jun;101(6):1373-1381. doi: 10.1189/jlb.4A0616-270R. Epub 2017 Mar 3. J Leukoc Biol. 2017. PMID: 28258153 Free PMC article.
-
Mucosal Immune Response in Nasal-Associated Lymphoid Tissue upon Intranasal Administration by Adjuvants.J Innate Immun. 2018;10(5-6):515-521. doi: 10.1159/000489405. Epub 2018 Jun 1. J Innate Immun. 2018. PMID: 29860261 Free PMC article. Review.
Cited by
-
The protective and pathogenic roles of IL-17 in viral infections: friend or foe?Open Biol. 2019 Jul 26;9(7):190109. doi: 10.1098/rsob.190109. Epub 2019 Jul 24. Open Biol. 2019. PMID: 31337278 Free PMC article. Review.
-
Mechanisms of Cholera Toxin in the Modulation of TH17 Responses.Crit Rev Immunol. 2015;35(2):135-52. doi: 10.1615/critrevimmunol.2015012295. Crit Rev Immunol. 2015. PMID: 26351147 Free PMC article. Review.
-
Ex Pluribus Unum: The CD4 T Cell Response against Influenza A Virus.Cells. 2024 Apr 5;13(7):639. doi: 10.3390/cells13070639. Cells. 2024. PMID: 38607077 Free PMC article. Review.
-
Correlation between interleukin gene polymorphisms and current prevalence and mortality rates due to novel coronavirus disease 2019 (COVID-2019) in 23 countries.J Med Virol. 2021 Oct;93(10):5853-5863. doi: 10.1002/jmv.27127. Epub 2021 Jun 10. J Med Virol. 2021. PMID: 34081354 Free PMC article.
-
Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection.Mucosal Immunol. 2020 Jan;13(1):64-74. doi: 10.1038/s41385-019-0206-9. Epub 2019 Oct 9. Mucosal Immunol. 2020. PMID: 31597930 Free PMC article.
References
-
- Neutra M.R., Kozlowski P.A. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. - PubMed
-
- Zygmunt B.M., Rharbaoui F., Groebe L., Guzman C.A. Intranasal immunization promotes Th17 immune responses. J Immunol. 2009;183:6933–6938. - PubMed
-
- Datta S.K., Sabet M., Nguyen K.P., Valdez P.A., Gonzalez-Navajas J.M., Islam S., Mihajlov I., Fierer J., Insel P.A., Webster N.J., Guiney D.G., Raz E. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc Natl Acad Sci USA. 2010;107:10638–10643. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

