New tides: using zebrafish to study renal regeneration
- PMID: 24183931
- PMCID: PMC3946610
- DOI: 10.1016/j.trsl.2013.10.003
New tides: using zebrafish to study renal regeneration
Abstract
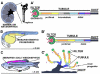
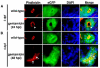
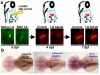
Over the past several decades, the zebrafish has become one of the major vertebrate model organisms used in biomedical research. In this arena, the zebrafish has emerged as an applicable system for the study of kidney diseases and renal regeneration. The relevance of the zebrafish model for nephrology research has been increasingly appreciated as the understanding of zebrafish kidney structure, ontogeny, and the response to damage has steadily expanded. Recent studies have documented the amazing regenerative characteristics of the zebrafish kidney, which include the ability to replace epithelial populations after acute injury and to grow new renal functional units, termed nephrons. Here we discuss how nephron composition is conserved between zebrafish and mammals, and highlight how recent findings from zebrafish studies utilizing transgenic technologies and chemical genetics can complement traditional murine approaches in the effort to dissect how the kidney responds to acute damage and identify therapeutics that enhance human renal regeneration.
Copyright © 2014 Mosby, Inc. All rights reserved.
Figures


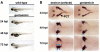
Similar articles
-
Analysis of nephron composition and function in the adult zebrafish kidney.J Vis Exp. 2014 Aug 9;(90):e51644. doi: 10.3791/51644. J Vis Exp. 2014. PMID: 25145398 Free PMC article.
-
Laser ablation of the zebrafish pronephros to study renal epithelial regeneration.J Vis Exp. 2011 Aug 29;(54):2845. doi: 10.3791/2845. J Vis Exp. 2011. PMID: 21897358 Free PMC article.
-
Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration.Wiley Interdiscip Rev Dev Biol. 2013 Sep-Oct;2(5):559-85. doi: 10.1002/wdev.92. Epub 2012 Oct 16. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24014448 Free PMC article. Review.
-
Characterization of mesonephric development and regeneration using transgenic zebrafish.Am J Physiol Renal Physiol. 2010 Nov;299(5):F1040-7. doi: 10.1152/ajprenal.00394.2010. Epub 2010 Sep 1. Am J Physiol Renal Physiol. 2010. PMID: 20810610 Free PMC article.
-
Little fish, big catch: zebrafish as a model for kidney disease.Kidney Int. 2016 Jun;89(6):1204-10. doi: 10.1016/j.kint.2016.01.031. Epub 2016 Apr 16. Kidney Int. 2016. PMID: 27165832 Free PMC article. Review.
Cited by
-
Zebrafish as a Model to Study Retinoic Acid Signaling in Development and Disease.Biomedicines. 2023 Apr 15;11(4):1180. doi: 10.3390/biomedicines11041180. Biomedicines. 2023. PMID: 37189798 Free PMC article. Review.
-
Zebrafish Renal Pathology: Emerging Models of Acute Kidney Injury.Curr Pathobiol Rep. 2015;3(2):171-181. doi: 10.1007/s40139-015-0082-2. Curr Pathobiol Rep. 2015. PMID: 25973344 Free PMC article. Review.
-
Zebrafish as a model for kidney function and disease.Pediatr Nephrol. 2019 May;34(5):751-762. doi: 10.1007/s00467-018-3921-7. Epub 2018 Mar 3. Pediatr Nephrol. 2019. PMID: 29502161 Free PMC article. Review.
-
Zebrafish models in translational research: tipping the scales toward advancements in human health.Dis Model Mech. 2014 Jul;7(7):739-43. doi: 10.1242/dmm.015545. Dis Model Mech. 2014. PMID: 24973743 Free PMC article. Review.
-
Using zebrafish to study podocyte genesis during kidney development and regeneration.Genesis. 2014 Sep;52(9):771-92. doi: 10.1002/dvg.22798. Epub 2014 Jun 25. Genesis. 2014. PMID: 24920186 Free PMC article. Review.
References
-
- Reilly RF, Bulger RE, Kriz W. Diseases of the Kidney and Urinary Tract. Lippincott Williams & Wilkins; Philadelphia: 2007. Structural-functional relationships in the kidney; pp. 2–53.
-
- Guyton AC, Hall JE. Textbook of Medical Physiology. Elsevier Saunders; Philadelphia: 2006. p. 310.
-
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. - PubMed
-
- Vize PD, Seufert DW, Carroll TJ, Wallingford JB. Model systems for the study of kidney development: use of the pronephros in the analysis of organ induction and patterning. Dev Biol. 1997;188:189–204. - PubMed
-
- Drummond I. Making a zebrafish kidney: a tale of two tubes. Trends Cell Biol. 2003;13:357–365. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

