Selective transport by SecA2: an expanding family of customized motor proteins
- PMID: 24184206
- PMCID: PMC4007388
- DOI: 10.1016/j.bbamcr.2013.10.019
Selective transport by SecA2: an expanding family of customized motor proteins
Abstract
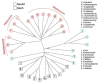
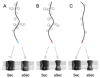
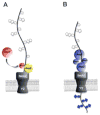
The SecA2 proteins are a special class of transport-associated ATPases that are related to the SecA component of the general Sec system, and are found in an increasingly large number of Gram-positive bacterial species. The SecA2 substrates are typically linked to the cell wall, but may be lipid-linked, peptidoglycan-linked, or non-covalently associated S-layer proteins. These substrates can have a significant impact on virulence of pathogenic organisms, but may also aid colonization by commensals. The SecA2 orthologues range from being highly similar to their SecA paralogues, to being distinctly different in apparent structure and function. Two broad classes of SecA2 are evident. One transports multiple substrates, and may interact with the general Sec system, or with an as yet unidentified transmembrane channel. The second type transports a single substrate, and is a component of the accessory Sec system, which includes the SecY paralogue SecY2 along with the accessory Sec proteins Asp1-3. Recent studies indicate that the latter three proteins may have a unique role in coordinating post-translational modification of the substrate with transport by SecA2. Comparative functional and phylogenetic analyses suggest that each SecA2 may be uniquely adapted for a specific type of substrate. This article is part of a Special Issue entitled: Protein trafficking and secretion in bacteria. Guest Editors: Anastassios Economou and Ross Dalbey.
Keywords: Accessory Sec system; Asp1; Asp2; Bacterial glycoprotein; Glycoprotein transport; S-layer.
© 2013.
Figures





References
-
- Bensing BA, Sullam PM. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol Microbiol. 2002;44:1081–94. - PubMed
-
- Lenz LL, Portnoy DA. Identification of a second Listeria secA gene associated with protein secretion and the rough phenotype. Mol Microbiol. 2002;45:1043–56. - PubMed
-
- Papanikolau Y, Papadovasilaki M, Ravelli RB, McCarthy AA, Cusack S, Economou A, Petratos K. Structure of dimeric SecA, the Escherichia coli preprotein translocase motor. J Mol Biol. 2007;366:1545–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

