Purification of a serine protease and evidence for a protein C activator from the saliva of the tick, Ixodes scapularis
- PMID: 24184517
- PMCID: PMC3877196
- DOI: 10.1016/j.toxicon.2013.10.025
Purification of a serine protease and evidence for a protein C activator from the saliva of the tick, Ixodes scapularis
Abstract
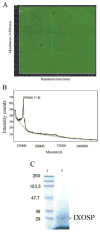
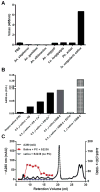
The saliva of ticks is critical to their survival as parasites and hematophagous animals. In this study, we have purified an enzyme with trypsin-like activity from the saliva of the tick vector of Lyme Disease, Ixodes scapularis. This enzyme, named as IXOSP (I. scapularis salivary serine protease), is a 29.9 kDa molecule with N-terminus FPxMVxLRIKxR. A BLAST search identified IXOSP as a secreted serine protease (AAY66740) with a conserved catalytic triad His, Asp, and Ser. In vitro studies demonstrated that IXOSP cleaves chromogenic substrates with arginine in the P1 position, by a mechanism inhibited by PMSF or aprotinin. Gene expression studies revealed that IXOSP is expressed at different tick developmental stages, including eggs, and unfed or fed adult tick salivary glands, but not in nymphs or in the midgut. While the physiological substrate for IXOSP remains to be identified, we demonstrated that I. scapularis saliva activate protein C (PC) resulting in the production of activated PC, a potent anticoagulant that also regulates a myriad of inflammatory responses through protease activated receptors. In contrast, the salivary glands of Anopheles gambiae, Anopheles stephensi, Anopheles albimanus, Aedes aegypti, Lutzomyia longipalpis, and Phlebotomus ariasi did not activate protein C. These discoveries are discussed in the context of blood coagulation, inflammation and vector-host interactions.
Keywords: Activated protein C (APC); Ixodes scapularis; Protease activated receptors (PAR); Saliva; Serine protease.
Published by Elsevier Ltd.
Conflict of interest statement
We certify that there is not conflict of interest for this paper
Figures

Similar articles
-
A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting.Int J Parasitol. 2014 May;44(6):369-79. doi: 10.1016/j.ijpara.2014.01.010. Epub 2014 Feb 28. Int J Parasitol. 2014. PMID: 24583183 Free PMC article.
-
Cloning of a salivary gland metalloprotease and characterization of gelatinase and fibrin(ogen)lytic activities in the saliva of the Lyme disease tick vector Ixodes scapularis.Biochem Biophys Res Commun. 2003 Jun 13;305(4):869-75. doi: 10.1016/s0006-291x(03)00857-x. Biochem Biophys Res Commun. 2003. PMID: 12767911 Free PMC article.
-
Tick saliva is a potent inhibitor of endothelial cell proliferation and angiogenesis.Thromb Haemost. 2005 Jul;94(1):167-74. doi: 10.1160/TH04-09-0566. Thromb Haemost. 2005. PMID: 16113800 Free PMC article.
-
A snapshot of the Ixodes scapularis degradome.Gene. 2011 Aug 15;482(1-2):78-93. doi: 10.1016/j.gene.2011.04.008. Epub 2011 Apr 28. Gene. 2011. PMID: 21596113 Free PMC article.
-
Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks.Ticks Tick Borne Dis. 2018 Mar;9(3):535-542. doi: 10.1016/j.ttbdis.2018.01.002. Epub 2018 Jan 31. Ticks Tick Borne Dis. 2018. PMID: 29398603 Free PMC article. Review.
Cited by
-
Ixodes scapularis Tick Saliva Proteins Sequentially Secreted Every 24 h during Blood Feeding.PLoS Negl Trop Dis. 2016 Jan 11;10(1):e0004323. doi: 10.1371/journal.pntd.0004323. eCollection 2016 Jan. PLoS Negl Trop Dis. 2016. PMID: 26751078 Free PMC article.
-
Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: a comparison between partially and fully engorged females.PLoS One. 2014 Apr 24;9(4):e94831. doi: 10.1371/journal.pone.0094831. eCollection 2014. PLoS One. 2014. PMID: 24762651 Free PMC article.
-
Changes in saliva protein profile throughout Rhipicephalus microplus blood feeding.Parasit Vectors. 2024 Jan 27;17(1):36. doi: 10.1186/s13071-024-06136-5. Parasit Vectors. 2024. PMID: 38281054 Free PMC article.
-
Borrelia burgdorferi infection modifies protein content in saliva of Ixodes scapularis nymphs.BMC Genomics. 2021 Mar 4;22(1):152. doi: 10.1186/s12864-021-07429-0. BMC Genomics. 2021. PMID: 33663385 Free PMC article.
-
Chemical Equilibrium at the Tick-Host Feeding Interface:A Critical Examination of Biological Relevance in Hematophagous Behavior.Front Physiol. 2019 May 1;10:530. doi: 10.3389/fphys.2019.00530. eCollection 2019. Front Physiol. 2019. PMID: 31118903 Free PMC article. Review.
References
-
- Amino R, Tanaka AS, Schenkman S. Triapsin, an unusual activatable serine protease from the saliva of the hematophagous vector of Chagas’ disease Triatoma infestans (Hemiptera: Reduviidae) Insect Biochem Mol Biol. 2001;31:465–472. - PubMed
-
- Ashida M, Kinoshita K, Brey PT. Studies on prophenoloxidase activation in the mosquito Aedes aegypti L. Eur J Biochem. 1990;188:507–515. - PubMed
-
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. - PubMed
-
- Collin N, Assumpcao TC, Mizurini DM, Gilmore DC, Dutra-Oliveira A, Kotsyfakis M, Sa-Nunes A, Teixeira C, Ribeiro JM, Monteiro RQ, Valenzuela JG, Francischetti IM. Lufaxin, a Novel Factor Xa Inhibitor From the Salivary Gland of the Sand Fly Lutzomyia longipalpis Blocks Protease-Activated Receptor 2 Activation and Inhibits Inflammation and Thrombosis In Vivo. Arterioscler Thromb Vasc Biol. 2012;32:2185–2198. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

