Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease
- PMID: 24184556
- PMCID: PMC3848617
- DOI: 10.4049/jimmunol.1102592
Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease
Abstract
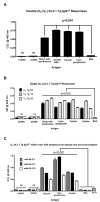
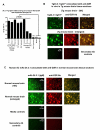
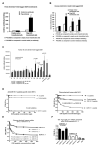
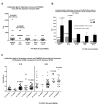
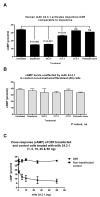
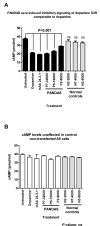
How autoantibodies target the brain and lead to disease in disorders such as Sydenham chorea (SC) is not known. SC is characterized by autoantibodies against the brain and is the main neurologic manifestation of streptococcal-induced rheumatic fever. Previously, our novel SC-derived mAb 24.3.1 was found to recognize streptococcal and brain Ags. To investigate in vivo targets of human mAb 24.3.1, VH/VL genes were expressed in B cells of transgenic (Tg) mice as functional chimeric human VH 24.3.1-mouse C-region IgG1(a) autoantibody. Chimeric human-mouse IgG1(a) autoantibody colocalized with tyrosine hydroxylase in the basal ganglia within dopaminergic neurons in vivo in VH 24.3.1 Tg mice. Both human mAb 24.3.1 and IgG1(a) in Tg sera were found to react with human dopamine D2 receptor (D2R). Reactivity of chorea-derived mAb 24.3.1 or SC IgG with D2R was confirmed by dose-dependent inhibitory signaling of D2R as a potential consequence of targeting dopaminergic neurons, reaction with surface-exposed FLAG epitope-tagged D2R, and blocking of Ab reactivity by an extracellular D2R peptide. IgG from SC and a related subset of streptococcal-associated behavioral disorders called "pediatric autoimmune neuropsychiatric disorder associated with streptococci" (PANDAS) with small choreiform movements reacted in ELISA with D2R. Reaction with FLAG-tagged D2R distinguished SC from PANDAS, whereas sera from both SC and PANDAS induced inhibitory signaling of D2R on transfected cells comparably to dopamine. In this study, we define a mechanism by which the brain may be altered by Ab in movement and behavioral disorders.
Figures

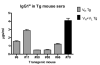


References
-
- Stollerman GH. Rheumatic fever in the 21st century. Clin Infect Dis. 2001;33:806–814. - PubMed
-
- Marques-Dias MJ, Mercadante MT, Tucker D, Lombroso P. Sydenham's chorea. Psychiatr Clin North Am. 1997;20:809–820. - PubMed
-
- Taranta A, Stollerman GH. The relationship of Sydenham's chorea to infection with group A streptococci. Am J Med. 1956;20:170–175. - PubMed
-
- Taranta A. Relation of isolated recurrences of Sydenham's chorea to preceding streptococcal infections. N Engl J Med. 1959;260:1204–1210. - PubMed
-
- Kurlan R, Kaplan EL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) etiology for tics and obsessive-compulsive symptoms: hypothesis or entity? Practical considerations for the clinician. Pediatrics. 2004;113:883–886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

