Thrombopoietin promotes NHEJ DNA repair in hematopoietic stem cells through specific activation of Erk and NF-κB pathways and their target, IEX-1
- PMID: 24184684
- PMCID: PMC4467873
- DOI: 10.1182/blood-2013-07-515874
Thrombopoietin promotes NHEJ DNA repair in hematopoietic stem cells through specific activation of Erk and NF-κB pathways and their target, IEX-1
Abstract
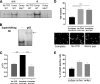
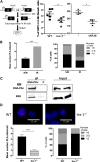
Loss of hematopoietic stem cell (HSC) function and increased risk of developing hematopoietic malignancies are severe and concerning complications of anticancer radiotherapy and chemotherapy. We have previously shown that thrombopoietin (TPO), a critical HSC regulator, ensures HSC chromosomal integrity and function in response to γ-irradiation by regulating their DNA-damage response. TPO directly affects the double-strand break (DSB) repair machinery through increased DNA-protein kinase (DNA-PK) phosphorylation and nonhomologous end-joining (NHEJ) repair efficiency and fidelity. This effect is not shared by other HSC growth factors, suggesting that TPO triggers a specific signal in HSCs facilitating DNA-PK activation upon DNA damage. The discovery of these unique signaling pathways will provide a means of enhancing TPO-desirable effects on HSCs and improving the safety of anticancer DNA agents. We show here that TPO specifically triggers Erk and nuclear factor κB (NF-κB) pathways in mouse hematopoietic stem and progenitor cells (HSPCs). Both of these pathways are required for a TPO-mediated increase in DSB repair. They cooperate to induce and activate the early stress-response gene, Iex-1 (ier3), upon DNA damage. Iex-1 forms a complex with pERK and the catalytic subunit of DNA-PK, which is necessary and sufficient to promote TPO-increased DNA-PK activation and NHEJ DSB repair in both mouse and human HSPCs.
Figures





Comment in
-
TPO signal for stem cell genomic integrity.Blood. 2014 Jan 23;123(4):459-60. doi: 10.1182/blood-2013-11-537084. Blood. 2014. PMID: 24458271
References
-
- Simonnet AJ, Nehmé J, Vaigot P, Barroca V, Leboulch P, Tronik-Le Roux D. Phenotypic and functional changes induced in hematopoietic stem/progenitor cells after gamma-ray radiation exposure. Stem Cells. 2009;27(6):1400–1409. - PubMed
-
- Iliakis G, Wang H, Perrault AR, et al. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet Genome Res. 2004;104(1-4):14–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

