A CREB3-ARF4 signalling pathway mediates the response to Golgi stress and susceptibility to pathogens
- PMID: 24185178
- PMCID: PMC3965854
- DOI: 10.1038/ncb2865
A CREB3-ARF4 signalling pathway mediates the response to Golgi stress and susceptibility to pathogens
Abstract
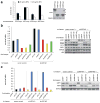
Treatment of cells with brefeldin A (BFA) blocks secretory vesicle transport and causes a collapse of the Golgi apparatus. To gain more insight into the cellular mechanisms mediating BFA toxicity, we conducted a genome-wide haploid genetic screen that led to the identification of the small G protein ADP-ribosylation factor 4 (ARF4). ARF4 depletion preserves viability, Golgi integrity and cargo trafficking in the presence of BFA, and these effects depend on the guanine nucleotide exchange factor GBF1 and other ARF isoforms including ARF1 and ARF5. ARF4 knockdown cells show increased resistance to several human pathogens including Chlamydia trachomatis and Shigella flexneri. Furthermore, ARF4 expression is induced when cells are exposed to several Golgi-disturbing agents and requires the CREB3 (also known as Luman or LZIP) transcription factor, whose downregulation mimics ARF4 loss. Thus, we have uncovered a CREB3-ARF4 signalling cascade that may be part of a Golgi stress response set in motion by stimuli compromising Golgi capacity.
Figures
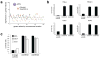





Similar articles
-
Brefeldin A and M-COPA block the export of RTKs from the endoplasmic reticulum via simultaneous inactivation of ARF1, ARF4, and ARF5.J Biol Chem. 2024 Jun;300(6):107327. doi: 10.1016/j.jbc.2024.107327. Epub 2024 Apr 26. J Biol Chem. 2024. PMID: 38679330 Free PMC article.
-
Characterization of class I and II ADP-ribosylation factors (Arfs) in live cells: GDP-bound class II Arfs associate with the ER-Golgi intermediate compartment independently of GBF1.Mol Biol Cell. 2008 Aug;19(8):3488-500. doi: 10.1091/mbc.e08-04-0373. Epub 2008 Jun 4. Mol Biol Cell. 2008. PMID: 18524849 Free PMC article.
-
Class II Arfs require a brefeldin-A-sensitive factor for Golgi association.Biochem Biophys Res Commun. 2020 Sep 10;530(1):301-306. doi: 10.1016/j.bbrc.2020.07.001. Epub 2020 Aug 6. Biochem Biophys Res Commun. 2020. PMID: 32828303
-
Activation of toxin ADP-ribosyltransferases by eukaryotic ADP-ribosylation factors.Mol Cell Biochem. 1999 Mar;193(1-2):153-7. Mol Cell Biochem. 1999. PMID: 10331652 Review.
-
The ins and outs of the Arf4-based ciliary membrane-targeting complex.Small GTPases. 2021 Jan;12(1):1-12. doi: 10.1080/21541248.2019.1616355. Epub 2019 May 17. Small GTPases. 2021. PMID: 31068062 Free PMC article. Review.
Cited by
-
Transcriptional regulation of secretory capacity by bZip transcription factors.Front Biol (Beijing). 2015 Feb 1;10(1):28-51. doi: 10.1007/s11515-014-1338-7. Front Biol (Beijing). 2015. PMID: 25821458 Free PMC article.
-
Changes in transcriptome of macrophages in atherosclerosis.J Cell Mol Med. 2015 Jun;19(6):1163-73. doi: 10.1111/jcmm.12591. Epub 2015 May 13. J Cell Mol Med. 2015. PMID: 25973901 Free PMC article. Review.
-
Vibrio splendidus AJ01 Promotes Pathogenicity via L-Glutamic Acid.Microorganisms. 2023 Sep 17;11(9):2333. doi: 10.3390/microorganisms11092333. Microorganisms. 2023. PMID: 37764177 Free PMC article.
-
Dissecting the Role of Disturbed ER-Golgi Trafficking in Antivirals and Alcohol Abuse-Induced Pathogenesis of Liver Disorders.J Drug Abuse. 2017;3(3):14. doi: 10.21767/2471-853X.100054. Epub 2017 Sep 26. J Drug Abuse. 2017. PMID: 29399658 Free PMC article.
-
Arfs on the Golgi: four conductors, one orchestra.Front Mol Biosci. 2025 Jul 31;12:1612531. doi: 10.3389/fmolb.2025.1612531. eCollection 2025. Front Mol Biosci. 2025. PMID: 40821699 Free PMC article. Review.
References
-
- Chiu VK, et al. Ras signalling on the endoplasmic reticulum and the Golgi. Nat Cell Biol. 2002;4:343–350. - PubMed
-
- Sutterlin C, Hsu P, Mallabiabarrena A, Malhotra V. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell. 2002;109:359–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

