Phase 2 trial of intravenously administered plerixafor for stem cell mobilization in patients with multiple myeloma following lenalidomide-based initial therapy
- PMID: 24185588
- PMCID: PMC3946357
- DOI: 10.1038/bmt.2013.175
Phase 2 trial of intravenously administered plerixafor for stem cell mobilization in patients with multiple myeloma following lenalidomide-based initial therapy
Abstract
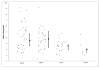
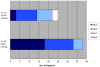
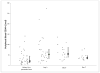
Initial therapy of multiple myeloma with lenalidomide-based regimens can compromise stem cell collection, which can be overcome with the addition of plerixafor. Plerixafor is typically given subcutaneously (SQ), with collection ∼11 h later for maximum yield. Intravenous administration may allow more rapid and predictable mobilization. This trial was designed to assess the efficacy and feasibility of IV plerixafor in patients receiving initial therapy with a lenalidomide-based regimen. Patients received G-CSF at 10 μg/kg/day for 4 days followed by IV plerixafor at 0.24 mg/kg/dose starting on day 5; plerixafor was administered early in the morning with apheresis 4-5 h later. Thirty-eight (97%) patients collected at least 3 × 10(6) CD34+ cells/kg within 2 days of apheresis. The median CD34+ cells/kg after 1 day of collection was 3.9 × 10(6) (range: 0.7-9.2) and after 2 days of collection was 6.99 × 10(6) (range: 1.1-16.5). There were no grade 3 or 4 non-hematological adverse events, and one patient experienced grade 4 thrombocytopenia. The most common adverse events were nausea, diarrhea and abdominal bloating. IV plerixafor is an effective strategy for mobilization with low failure rate and is well tolerated. It offers flexibility with a schedule of early-morning infusion followed by apheresis later in the day.
Figures
Similar articles
-
Peripheral blood stem cell mobilization in multiple myeloma patients treat in the novel therapy-era with plerixafor and G-CSF has superior efficacy but significantly higher costs compared to mobilization with low-dose cyclophosphamide and G-CSF.J Clin Apher. 2013 Oct;28(5):359-67. doi: 10.1002/jca.21280. Epub 2013 Jun 14. J Clin Apher. 2013. PMID: 23765597
-
Plerixafor for autologous peripheral blood stem cell mobilization in patients previously treated with fludarabine or lenalidomide.Biol Blood Marrow Transplant. 2012 Feb;18(2):314-7. doi: 10.1016/j.bbmt.2011.10.003. Epub 2011 Oct 13. Biol Blood Marrow Transplant. 2012. PMID: 22001752 Clinical Trial.
-
Stem Cell Mobilization Yields with Daratumumab- and Lenalidomide-Containing Quadruplet Induction Therapy in Newly Diagnosed Multiple Myeloma: Findings from the MASTER and GRIFFIN Trials.Transplant Cell Ther. 2023 Mar;29(3):174.e1-174.e10. doi: 10.1016/j.jtct.2022.11.029. Epub 2022 Dec 6. Transplant Cell Ther. 2023. PMID: 36494017
-
Plerixafor: A chemokine receptor-4 antagonist for mobilization of hematopoietic stem cells for transplantation after high-dose chemotherapy for non-Hodgkin's lymphoma or multiple myeloma.Clin Ther. 2010 May;32(5):821-43. doi: 10.1016/j.clinthera.2010.05.007. Clin Ther. 2010. PMID: 20685493 Review.
-
Plerixafor: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma.Drugs. 2011 Aug 20;71(12):1623-47. doi: 10.2165/11206040-000000000-00000. Drugs. 2011. PMID: 21861545 Review.
Cited by
-
Prospective identification of potential factors influencing stem cell mobilization and the necessity for plerixafor use in newly diagnosed multiple myeloma patients undergoing autologous stem cell transplantation.Hematol Transfus Cell Ther. 2021 Oct-Dec;43(4):402-409. doi: 10.1016/j.htct.2020.04.011. Epub 2020 Jul 23. Hematol Transfus Cell Ther. 2021. PMID: 32792260 Free PMC article.
-
Molecular Pathways: Deciphering Mechanisms of Resistance to Macrophage-Targeted Therapies.Clin Cancer Res. 2017 Feb 15;23(4):876-884. doi: 10.1158/1078-0432.CCR-16-0133. Epub 2016 Nov 28. Clin Cancer Res. 2017. PMID: 27895033 Free PMC article. Review.
-
Comparison of efficacy, safety, patients' quality of life, and doctors' occupational stress between lenalidomide-based and bortezomib-based induction in patients with newly diagnosed multiple myeloma.Cancer Med. 2021 Mar;10(5):1656-1667. doi: 10.1002/cam4.3762. Epub 2021 Feb 2. Cancer Med. 2021. PMID: 33527753 Free PMC article. Clinical Trial.
-
How we manage autologous stem cell transplantation for patients with multiple myeloma.Blood. 2014 Aug 7;124(6):882-90. doi: 10.1182/blood-2014-03-544759. Epub 2014 Jun 27. Blood. 2014. PMID: 24973360 Free PMC article. Review.
-
The Efficacy and Safety of Chemotherapy-Based Stem Cell Mobilization in Multiple Myeloma Patients Who Are Poor Responders to Induction: The Mayo Clinic Experience.Transplant Cell Ther. 2021 Sep;27(9):770.e1-770.e7. doi: 10.1016/j.jtct.2021.06.016. Epub 2021 Jun 18. Transplant Cell Ther. 2021. PMID: 34153504 Free PMC article.
References
-
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–7. - PubMed
-
- Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. The New England journal of medicine. 2003;348(19):1875–83. - PubMed
-
- Kumar S. Multiple myeloma - current issues and controversies. Cancer Treat Rev. 2010;36 (Suppl 2):S3–11. - PubMed
-
- Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100) Leukemia. 2009;23(10):1904–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

