Biology of the TAM receptors
- PMID: 24186067
- PMCID: PMC3809585
- DOI: 10.1101/cshperspect.a009076
Biology of the TAM receptors
Abstract
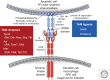
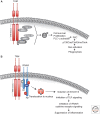
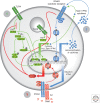
The TAM receptors--Tyro3, Axl, and Mer--comprise a unique family of receptor tyrosine kinases, in that as a group they play no essential role in embryonic development. Instead, they function as homeostatic regulators in adult tissues and organ systems that are subject to continuous challenge and renewal throughout life. Their regulatory roles are prominent in the mature immune, reproductive, hematopoietic, vascular, and nervous systems. The TAMs and their ligands--Gas6 and Protein S--are essential for the efficient phagocytosis of apoptotic cells and membranes in these tissues; and in the immune system, they act as pleiotropic inhibitors of the innate inflammatory response to pathogens. Deficiencies in TAM signaling are thought to contribute to chronic inflammatory and autoimmune disease in humans, and aberrantly elevated TAM signaling is strongly associated with cancer progression, metastasis, and resistance to targeted therapies.
Figures
References
-
- Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, et al. 2008. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol 28: 1429–1431 - PubMed
-
- Akira S 2006. TLR signaling. Curr Top Microbiol Immunol 311: 1–16 - PubMed
-
- Allen MP, Xu M, Linseman DA, Pawlowski JE, Bokoch GM, Heidenreich KA, Wierman ME 2002b. Adhesion-related kinase repression of gonadotropin-releasing hormone gene expression requires Rac activation of the extracellular signal-regulated kinase pathway. J Biol Chem 277: 38133–38140 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
