Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease
- PMID: 24186072
- PMCID: PMC3809581
- DOI: 10.1101/cshperspect.a021220
Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease
Abstract
The unorthodox genetics of the mtDNA is providing new perspectives on the etiology of the common "complex" diseases. The maternally inherited mtDNA codes for essential energy genes, is present in thousands of copies per cell, and has a very high mutation rate. New mtDNA mutations arise among thousands of other mtDNAs. The mechanisms by which these "heteroplasmic" mtDNA mutations come to predominate in the female germline and somatic tissues is poorly understood, but essential for understanding the clinical variability of a range of diseases. Maternal inheritance and heteroplasmy also pose major challengers for the diagnosis and prevention of mtDNA disease.
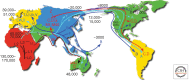
Figures





References
-
- Babu GJ, Feigelson ED 2006. Astrostatistics: Goodness-of-fit and all that! Astronomical Data Analysis Software and Systems XV ASP Conference Series 351: 127
-
- Ballinger SW, Shoffner JM, Hedaya EV, Trounce I, Polak MA, Koontz DA, Wallace DC 1992. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet 1: 11–15 - PubMed
-
- Ballinger SW, Shoffner JM, Gebhart S, Koontz DA, Wallace DC 1994. Mitochondrial diabetes revisited. Nat Genet 7: 458–459 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
