Role of ASIC1 in the development of chronic hypoxia-induced pulmonary hypertension
- PMID: 24186095
- PMCID: PMC3920158
- DOI: 10.1152/ajpheart.00269.2013
Role of ASIC1 in the development of chronic hypoxia-induced pulmonary hypertension
Abstract
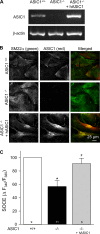
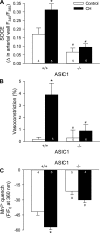
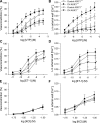
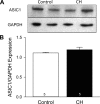
Chronic hypoxia (CH) associated with respiratory disease results in elevated pulmonary vascular intracellular Ca(2+) concentration, which elicits enhanced vasoconstriction and promotes vascular arterial remodeling and thus has important implications in the development of pulmonary hypertension (PH). Store-operated Ca(2+) entry (SOCE) contributes to this elevated intracellular Ca(2+) concentration and has also been linked to acute hypoxic pulmonary vasoconstriction (HPV). Since our laboratory has recently demonstrated an important role for acid-sensing ion channel 1 (ASIC1) in mediating SOCE, we hypothesized that ASIC1 contributes to both HPV and the development of CH-induced PH. To test this hypothesis, we examined responses to acute hypoxia in isolated lungs and assessed the effects of CH on indexes of PH, arterial remodeling, and vasoconstrictor reactivity in wild-type (ASIC1(+/+)) and ASIC1 knockout (ASIC1(-/-)) mice. Restoration of ASIC1 expression in pulmonary arterial smooth muscle cells from ASIC1(-/-) mice rescued SOCE, confirming the requirement for ASIC1 in this response. HPV responses were blunted in lungs from ASIC1(-/-) mice. Both SOCE and receptor-mediated Ca(2+) entry, along with agonist-dependent vasoconstrictor responses, were diminished in small pulmonary arteries from control ASIC(-/-) mice compared with ASIC(+/+) mice. The effects of CH to augment receptor-mediated vasoconstrictor and SOCE responses in vessels from ASIC1(+/+) mice were not observed after CH in ASIC1(-/-) mice. In addition, ASIC1(-/-) mice exhibited diminished right ventricular systolic pressure, right ventricular hypertrophy, and arterial remodeling in response to CH compared with ASIC1(+/+) mice. Taken together, these data demonstrate an important role for ASIC1 in both HPV and the development of CH-induced PH.
Keywords: acid-sensing ion channels; capacitative Ca2+ entry; degenerin/epithelial Na+ channel; hypoxic pulmonary vasoconstriction; pulmonary vascular remodeling; receptor-mediated vasoconstriction; store-operated Ca2+ entry.
Figures



Similar articles
-
Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2014 Sep 1;307(5):L419-30. doi: 10.1152/ajplung.00095.2014. Epub 2014 Jul 3. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24993130 Free PMC article.
-
Loss of acid-sensing ion channel 2 enhances pulmonary vascular resistance and hypoxic pulmonary hypertension.J Appl Physiol (1985). 2019 Aug 1;127(2):393-407. doi: 10.1152/japplphysiol.00894.2018. Epub 2019 Jun 6. J Appl Physiol (1985). 2019. PMID: 31169471 Free PMC article.
-
Chronic hypoxia upregulates pulmonary arterial ASIC1: a novel mechanism of enhanced store-operated Ca2+ entry and receptor-dependent vasoconstriction.Am J Physiol Cell Physiol. 2012 Mar 15;302(6):C931-40. doi: 10.1152/ajpcell.00332.2011. Epub 2011 Dec 28. Am J Physiol Cell Physiol. 2012. PMID: 22205392 Free PMC article.
-
Smooth muscle acid-sensing ion channel 1: pathophysiological implication in hypoxic pulmonary hypertension.Exp Physiol. 2015 Feb 1;100(2):111-20. doi: 10.1113/expphysiol.2014.081612. Epub 2015 Jan 14. Exp Physiol. 2015. PMID: 25398716 Review.
-
Hypoxic pulmonary vasoconstriction: role of ion channels.J Appl Physiol (1985). 2005 Jan;98(1):415-20. doi: 10.1152/japplphysiol.00732.2004. J Appl Physiol (1985). 2005. PMID: 15591311 Review.
Cited by
-
Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension.PLoS One. 2017 Jun 30;12(6):e0180455. doi: 10.1371/journal.pone.0180455. eCollection 2017. PLoS One. 2017. PMID: 28666030 Free PMC article.
-
Alveolar nonselective channels are ASIC1a/α-ENaC channels and contribute to AFC.Am J Physiol Lung Cell Mol Physiol. 2017 Jun 1;312(6):L797-L811. doi: 10.1152/ajplung.00379.2016. Epub 2017 Mar 10. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28283476 Free PMC article.
-
Update on novel targets and potential treatment avenues in pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2016 Nov 1;311(5):L811-L831. doi: 10.1152/ajplung.00302.2016. Epub 2016 Sep 2. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27591245 Free PMC article. Review.
-
ASIC1-mediated calcium entry stimulates NFATc3 nuclear translocation via PICK1 coupling in pulmonary arterial smooth muscle cells.Am J Physiol Lung Cell Mol Physiol. 2016 Jul 1;311(1):L48-58. doi: 10.1152/ajplung.00040.2016. Epub 2016 May 17. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27190058 Free PMC article.
-
Identification of candidate biomarkers and pathways associated with type 1 diabetes mellitus using bioinformatics analysis.Sci Rep. 2022 Jun 1;12(1):9157. doi: 10.1038/s41598-022-13291-1. Sci Rep. 2022. PMID: 35650387 Free PMC article.
References
-
- Berdiev BK, Xia J, Jovov B, Markert JM, Mapstone TB, Gillespie GY, Fuller CM, Bubien JK, Benos DJ. Protein kinase C isoform antagonism controls BNaC2 (ASIC1) function. J Biol Chem 277: 45734–45740, 2002 - PubMed
-
- Chu X, Cheung JY, Barber DL, Birnbaumer L, Rothblum LI, Conrad K, Abrasonis V, Chan YM, Stahl R, Carey DJ, Miller BA. Erythropoietin modulates calcium influx through TRPC2. J Biol Chem 277: 34375–34382, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

