Cardiomyocyte Ogt is essential for postnatal viability
- PMID: 24186210
- PMCID: PMC3920156
- DOI: 10.1152/ajpheart.00438.2013
Cardiomyocyte Ogt is essential for postnatal viability
Abstract
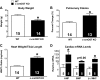
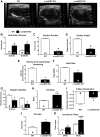
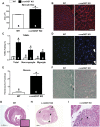
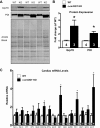
The singly coded gene O-linked-β-N-acetylglucosamine (O-GlcNAc) transferase (Ogt) resides on the X chromosome and is necessary for embryonic stem cell viability during embryogenesis. In mature cells, this enzyme catalyzes the posttranslational modification known as O-GlcNAc to various cellular proteins. Several groups, including our own, have shown that acute increases in protein O-GlcNAcylation are cardioprotective both in vitro and in vivo. Yet, little is known about how OGT affects cardiac function because total body knockout (KO) animals are not viable. Presently, we sought to establish the potential involvement of cardiomyocyte Ogt in cardiac maturation. Initially, we characterized a constitutive cardiomyocyte-specific (cm)OGT KO (c-cmOGT KO) mouse and found that only 12% of the c-cmOGT KO mice survived to weaning age (4 wk old); the surviving animals were smaller than their wild-type littermates, had dilated hearts, and showed overt signs of heart failure. Dysfunctional c-cmOGT KO hearts were more fibrotic, apoptotic, and hypertrophic. Several glycolytic genes were also upregulated; however, there were no gross changes in mitochondrial O2 consumption. Histopathology of the KO hearts indicated the potential involvement of endoplasmic reticulum stress, directing us to evaluate expression of 78-kDa glucose-regulated protein and protein disulfide isomerase, which were elevated. Additional groups of mice were subjected to inducible deletion of cmOGT, which did not produce overt dysfunction within the first couple of weeks of deletion. Yet, long-term loss (via inducible deletion) of cmOGT produced gradual and progressive cardiomyopathy. Thus, cardiomyocyte Ogt is necessary for maturation of the mammalian heart, and inducible deletion of cmOGT in the adult mouse produces progressive ventricular dysfunction.
Keywords: O-linked-β-N-acetylglucosamine transferase; cardiac function; hypertrophy; metabolism; remodeling; uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetylglucosaminyltransferase.
Figures


References
-
- Belke DD. Swim-exercised mice show a decreased level of protein O-GlcNAcylation and expression of O-GlcNAc transferase in heart. J Appl Physiol 111: 157–162, 2011 - PubMed
-
- Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 29: 2831–2842, 2010 - PubMed
-
- Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol 291: C178–C187, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

