Major epigenetic development distinguishing neuronal and non-neuronal cells occurs postnatally in the murine hypothalamus
- PMID: 24186871
- PMCID: PMC3929094
- DOI: 10.1093/hmg/ddt548
Major epigenetic development distinguishing neuronal and non-neuronal cells occurs postnatally in the murine hypothalamus
Abstract
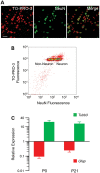
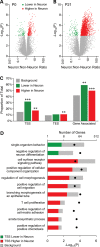
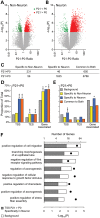
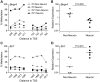
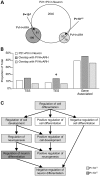
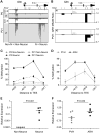
Prenatal and early postnatal environment can persistently alter one's risk of obesity. Environmental effects on hypothalamic developmental epigenetics constitute a likely mechanism underlying such 'developmental programming' of energy balance regulation. To advance our understanding of these processes, it is essential to develop approaches to disentangle the cellular and regional heterogeneity of hypothalamic developmental epigenetics. We therefore performed genome-scale DNA methylation profiling in hypothalamic neurons and non-neuronal cells at postnatal day 0 (P0) and P21 and found, surprisingly, that most of the DNA methylation differences distinguishing these two cell types are established postnatally. In particular, neuron-specific increases in DNA methylation occurred extensively at genes involved in neuronal development. Quantitative bisulfite pyrosequencing verified our methylation profiling results in all 15 regions examined, and expression differences were associated with DNA methylation at several genes. We also identified extensive methylation differences between the arcuate (ARH) and paraventricular nucleus of the hypothalamus (PVH). Integrating these two data sets showed that genomic regions with PVH versus ARH differential methylation strongly overlap with those undergoing neuron-specific increases from P0 to P21, suggesting that these developmental changes occur preferentially in either the ARH or PVH. In particular, neuron-specific methylation increases at the 3' end of Shh localized to the ARH and were positively associated with gene expression. Our data indicate a key role for DNA methylation in establishing the gene expression potential of diverse hypothalamic cell types, and provide the novel insight that early postnatal life is a critical period for cell type-specific epigenetic development in the murine hypothalamus.
Figures

References
-
- Atkinson R.L., Pietrobelli A., Uauy R., Macdonald I.A. Are we attacking the wrong targets in the fight against obesity?: the importance of intervention in women of childbearing age. Int. J. Obes. (Lond) 2012;36: 1259–1260. - PubMed
-
- Gluckman P.D., Hanson M., Zimmet P., Forrester T. Losing the war against obesity: the need for a developmental perspective. Sci. Trans. Med. 2011;3 1–4. - PubMed
-
- Gluckman P.D., Hanson M.A., Buklijas T., Low F.M., Beedle A.S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009;5:401–408. - PubMed
-
- Waterland R.A., Garza C. Potential mechanisms of metabolic imprinting that lead to chronic disease. Am. J. Clin. Nutr. 1999;69:179–197. - PubMed
-
- Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33(Suppl):245–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

