Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes
- PMID: 24186878
- PMCID: PMC3836134
- DOI: 10.2337/dc13-0663
Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes
Abstract
Objective: To investigate the long-term safety and efficacy of empagliflozin, a sodium glucose cotransporter 2 inhibitor; sitagliptin; and metformin in patients with type 2 diabetes.
Research design and methods: In this randomized, open-label, 78-week extension study of two 12-week, blinded, dose-finding studies of empagliflozin (monotherapy and add-on to metformin) with open-label comparators, 272 patients received 10 mg empagliflozin (166 as add-on to metformin), 275 received 25 mg empagliflozin (166 as add-on to metformin), 56 patients received metformin, and 56 patients received sitagliptin as add-on to metformin.
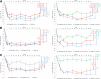
Results: Changes from baseline in HbA1c at week 90 were -0.34 to -0.63% (-3.7 to -6.9 mmol/mol) with empagliflozin, -0.56% (-6.1 mmol/mol) with metformin, and -0.40% (-4.4 mmol/mol) with sitagliptin. Changes from baseline in weight at week 90 were -2.2 to -4.0 kg with empagliflozin, -1.3 kg with metformin, and -0.4 kg with sitagliptin. Adverse events (AEs) were reported in 63.2-74.1% of patients on empagliflozin and 69.6% on metformin or sitagliptin; most AEs were mild or moderate in intensity. Hypoglycemic events were rare in all treatment groups, and none required assistance. AEs consistent with genital infections were reported in 3.0-5.5% of patients on empagliflozin, 1.8% on metformin, and none on sitagliptin. AEs consistent with urinary tract infections were reported in 3.8-12.7% of patients on empagliflozin, 3.6% on metformin, and 12.5% on sitagliptin.
Conclusions: Long-term empagliflozin treatment provided sustained glycemic and weight control and was well tolerated with a low risk of hypoglycemia in patients with type 2 diabetes.
Trial registration: ClinicalTrials.gov NCT00881530.
Figures
References
-
- Campbell RK. Fate of the beta-cell in the pathophysiology of type 2 diabetes. J Am Pharm Assoc (2003) 2009;49(Suppl. 1):S10–S15 - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 - PubMed
-
- Turner RC, Cull CA, Frighi V, Holman RR, UK Prospective Diabetes Study (UKPDS) Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 - PubMed
-
- Alvarez Guisasola F, Mavros P, Nocea G, Alemao E, Alexander CM, Yin D. Glycaemic control among patients with type 2 diabetes mellitus in seven European countries: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) study. Diabetes Obes Metab 2008;10(Suppl. 1):8–15 - PubMed
-
- Aguilar RB. Evaluating treatment algorithms for the management of patients with type 2 diabetes mellitus: a perspective on the definition of treatment success. Clin Ther 2011;33:408–424 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

