Structure and function of Norrin in assembly and activation of a Frizzled 4-Lrp5/6 complex
- PMID: 24186977
- PMCID: PMC3828517
- DOI: 10.1101/gad.228544.113
Structure and function of Norrin in assembly and activation of a Frizzled 4-Lrp5/6 complex
Abstract
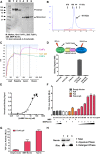
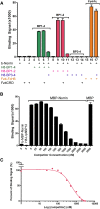
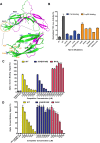
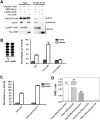
Norrin is a cysteine-rich growth factor that is required for angiogenesis in the eye, ear, brain, and female reproductive organs. It functions as an atypical Wnt ligand by specifically binding to the Frizzled 4 (Fz4) receptor. Here we report the crystal structure of Norrin, which reveals a unique dimeric structure with each monomer adopting a conserved cystine knot fold. Functional studies demonstrate that the novel Norrin dimer interface is required for Fz4 activation. Furthermore, we demonstrate that Norrin contains separate binding sites for Fz4 and for the Wnt ligand coreceptor Lrp5 (low-density lipoprotein-related protein 5) or Lrp6. Instead of inducing Fz4 dimerization, Norrin induces the formation of a ternary complex with Fz4 and Lrp5/6 by binding to their respective extracellular domains. These results provide crucial insights into the assembly and activation of the Norrin-Fz4-Lrp5/6 signaling complex.
Keywords: Frizzled 4; Norrin structure; Wnt/β-catenin signaling; cystine knot growth factor; low-density lipoprotein receptor-related protein 5/6; tetraspanin 12.
Figures


References
-
- Bailey RL, Herbert JM, Khan K, Heath VL, Bicknell R, Tomlinson MG 2011. The emerging role of tetraspanin microdomains on endothelial cells. Biochem Soc Trans 39: 1667–1673 - PubMed
-
- Berger W 1998. Molecular dissection of Norrie disease. Acta Anat (Basel) 162: 95–100 - PubMed
-
- Bourhis E, Wang W, Tam C, Hwang J, Zhang Y, Spittler D, Huang OW, Gong Y, Estevez A, Zilberleyb I, et al. 2011. Wnt antagonists bind through a short peptide to the first β-propeller domain of LRP5/6. Structure 19: 1433–1442 - PubMed
-
- Bradford MM 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
-
- Chen ZY, Battinelli EM, Fielder A, Bundey S, Sims K, Breakefield XO, Craig IW 1993. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet 5: 180–183 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
