σK of Clostridium acetobutylicum is the first known sporulation-specific sigma factor with two developmentally separated roles, one early and one late in sporulation
- PMID: 24187083
- PMCID: PMC3911250
- DOI: 10.1128/JB.01103-13
σK of Clostridium acetobutylicum is the first known sporulation-specific sigma factor with two developmentally separated roles, one early and one late in sporulation
Abstract
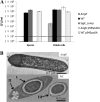
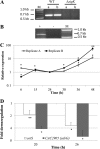
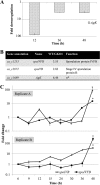
Sporulation in the model endospore-forming organism Bacillus subtilis proceeds via the sequential and stage-specific activation of the sporulation-specific sigma factors, σ(H) (early), σ(F), σ(E), σ(G), and σ(K) (late). Here we show that the Clostridium acetobutylicum σ(K) acts both early, prior to Spo0A expression, and late, past σ(G) activation, thus departing from the B. subtilis model. The C. acetobutylicum sigK deletion (ΔsigK) mutant was unable to sporulate, and solventogenesis, the characteristic stationary-phase phenomenon for this organism, was severely diminished. Transmission electron microscopy demonstrated that the ΔsigK mutant does not develop an asymmetric septum and produces no granulose. Complementation of sigK restored sporulation and solventogenesis to wild-type levels. Spo0A and σ(G) proteins were not detectable by Western analysis, while σ(F) protein levels were significantly reduced in the ΔsigK mutant. spo0A, sigF, sigE, sigG, spoIIE, and adhE1 transcript levels were all downregulated in the ΔsigK mutant, while those of the sigH transcript were unaffected during the exponential and transitional phases of culture. These data show that σ(K) is necessary for sporulation prior to spo0A expression. Plasmid-based expression of spo0A in the ΔsigK mutant from a nonnative promoter restored solventogenesis and the production of Spo0A, σ(F), σ(E), and σ(G), but not sporulation, which was blocked past the σ(G) stage of development, thus demonstrating that σ(K) is also necessary in late sporulation. sigK is expressed very early at low levels in exponential phase but is strongly upregulated during the middle to late stationary phase. This is the first sporulation-specific sigma factor shown to have two developmentally separated roles.
Figures




References
-
- Nolling J, Breton G, Omelchenko MV, Makarova KS, Zeng QD, Gibson R, Lee HM, Dubois J, Qiu DY, Hitti J, GTC Sequencing Center Production, Finishing, and Bioinformatics Teams. Wolf YI, Tatusov RL, Sabathe F, Doucette-Stamm L, Soucaille P, Daly MJ, Bennett GN, Koonin EV, Smith DR, . 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823–4838. 10.1128/JB.183.16.4823-4838.2001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

