The structure of integrin α1I domain in complex with a collagen-mimetic peptide
- PMID: 24187131
- PMCID: PMC3873540
- DOI: 10.1074/jbc.M113.480251
The structure of integrin α1I domain in complex with a collagen-mimetic peptide
Abstract
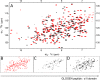
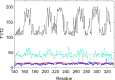
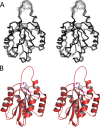
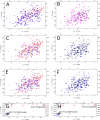
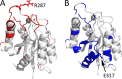
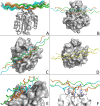
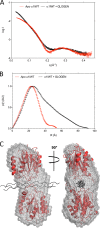
We have determined the structure of the human integrin α1I domain bound to a triple-helical collagen peptide. The structure of the α1I-peptide complex was investigated using data from NMR, small angle x-ray scattering, and size exclusion chromatography that were used to generate and validate a model of the complex using the data-driven docking program, HADDOCK (High Ambiguity Driven Biomolecular Docking). The structure revealed that the α1I domain undergoes a major conformational change upon binding of the collagen peptide. This involves a large movement in the C-terminal helix of the αI domain that has been suggested to be the mechanism by which signals are propagated in the intact integrin receptor. The structure suggests a basis for the different binding selectivity observed for the α1I and α2I domains. Mutational data identify residues that contribute to the conformational change observed. Furthermore, small angle x-ray scattering data suggest that at low collagen peptide concentrations the complex exists in equilibrium between a 1:1 and 2:1 α1I-peptide complex.
Keywords: Collagen; Extracellular Matrix; Integrins; NMR; Protein Structure; Protein-Protein Interactions.
Figures


References
-
- Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 - PubMed
-
- Gullberg D. E., Lundgren-Akerlund E. (2002) Collagen-binding I domain integrins—what do they do? Prog. Histochem. Cytochem. 37, 3–54 - PubMed
-
- Gullberg D. E. (ed) (2003) I Domains in Integrins, pp. 25–57, Landes Bioscience, Georgetown, TX
-
- Eble J. A. (2005) Collagen-binding integrins as pharmaceutical targets. Curr. Pharm. Des. 11, 867–880 - PubMed
-
- Vanderslice P., Woodside D. G. (2006) Integrin antagonists as therapeutics for inflammatory diseases. Expert Opin. Investig. Drugs 15, 1235–1255 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

