In vitro activation and inhibition of recombinant EGFR tyrosine kinase expressed in Escherichia coli
- PMID: 24187524
- PMCID: PMC3800664
- DOI: 10.1155/2013/807284
In vitro activation and inhibition of recombinant EGFR tyrosine kinase expressed in Escherichia coli
Abstract
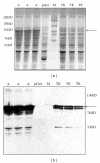
The present work concerns the heterologous expression of the intracellular domain harbouring the tyrosine kinase activity of the epidermal growth factor receptor (EGFR). Protein expression was improved thanks to the deletion of a 13-amino acid peptide of the juxtamembrane region (JM). The recombinant proteins were produced as a glutathione S-transferase (GST) fusion in Escherichia coli, and the solubilisation was performed by sarkosyl addition during extraction. The produced proteins spontaneously dimerize allowing the activation of the tyrosine kinase domain in the presence of [γ-(32)P]ATP. The activity assay has revealed the autophosphorylation of EGFR proteins which was decreased in the presence of genistein. Our system could facilitate the screening of EGFR inhibitors without the need of adding an exogenous substrate.
Figures


Similar articles
-
Expression of epidermal growth factor receptor sequences as E. coli fusion proteins: applications in the study of tyrosine kinase function.Biochem Biophys Res Commun. 1990 Jan 15;166(1):90-100. doi: 10.1016/0006-291x(90)91915-f. Biochem Biophys Res Commun. 1990. PMID: 2154210
-
Interactions between the juxtamembrane domain of the EGFR and calmodulin measured by surface plasmon resonance.Cell Signal. 2002 Dec;14(12):1005-13. doi: 10.1016/s0898-6568(02)00034-7. Cell Signal. 2002. PMID: 12359306
-
Epidermal growth factor receptor juxtamembrane region regulates allosteric tyrosine kinase activation.Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19238-43. doi: 10.1073/pnas.0703854104. Epub 2007 Nov 27. Proc Natl Acad Sci U S A. 2007. PMID: 18042729 Free PMC article.
-
Epidermal growth factor receptor as a therapeutic target in glioblastoma.Neuromolecular Med. 2013 Jun;15(2):420-34. doi: 10.1007/s12017-013-8229-y. Epub 2013 Apr 11. Neuromolecular Med. 2013. PMID: 23575987 Review.
-
Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review.
Cited by
-
A premature termination of human epidermal growth factor receptor transcription in Escherichia coli.ScientificWorldJournal. 2014;2014:830923. doi: 10.1155/2014/830923. Epub 2014 Oct 15. ScientificWorldJournal. 2014. PMID: 25389535 Free PMC article.
-
Soy-Based Therapeutic Baby Formulas: Testable Hypotheses Regarding the Pros and Cons.Front Nutr. 2017 Jan 18;3:59. doi: 10.3389/fnut.2016.00059. eCollection 2016. Front Nutr. 2017. PMID: 28149839 Free PMC article.
References
-
- Hamid O. Emerging treatments in oncology: focus on tyrosine kinase (erbB) receptor inhibitors. Journal of the American Pharmacists Association. 2004;44(1):52–58. - PubMed
-
- Burgess AW, Cho H-S, Eigenbrot C, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Molecular Cell. 2003;12(3):541–552. - PubMed
-
- Elloumi J, Jellali K, Jemel I, Aifa S. Monoclonal antibodies as cancer therapeutics. Recent Patents on Biotechnology. 2012;6(1):45–56. - PubMed
-
- Jemel I, Jellali K, Elloumi J, Aifa S. The offer of chemistry to targeted therapy in cancer. Recent Patents on Biotechnology. 2011;5(3):174–182. - PubMed
-
- Koland JG, Maddison O’Brien K, Cerione RA. Expression of epidermal growth factor receptor sequences as E. coli fusion proteins: applications in the study of tyrosine kinase function. Biochemical and Biophysical Research Communications. 1990;166(1):90–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

