The effect of gold and iron-oxide nanoparticles on biofilm-forming pathogens
- PMID: 24187645
- PMCID: PMC3800661
- DOI: 10.1155/2013/272086
The effect of gold and iron-oxide nanoparticles on biofilm-forming pathogens
Abstract
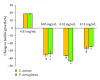
Microbial biofilms on biomaterial implants or devices are hard to eliminate by antibiotics due to their protection by exopolymeric substances that embed the organisms in a matrix, impenetrable for most antibiotics and immune-cells. Application of metals in their nanoparticulated form is currently considered to resolve bacterial infections. Gold and iron-oxide nanoparticles are widely used in different medical applications, but their utilisation to eradicate biofilms on biomaterials implants is novel. Here, we studied the effect of gold and iron oxide nanoparticles on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. We report that biofilm growth was reduced at higher concentrations of gold and iron-oxide nanoparticles compared to absence of nanoparticles. Thus nanoparticles with appropriate concentration could show significant reduction in biofilm formation.
Figures




References
-
- Costerton JW. Introduction to biofilm. International Journal of Antimicrobial Agents. 1999;11(3-4):217–221. - PubMed
-
- Gradinger R, Graf R, Grifka J, Löhr J. Das infezierte implantat. Der Orthopäde. 2008;3:257–269. - PubMed
-
- Mehta A, Rosenthal VD, Mehta Y, et al. Device-associated nosocomial infection rates in intensive care units of seven Indian cities. Findings of the international nosocomial infection control consortium (INICC) Journal of Hospital Infection. 2007;67(2):168–174. - PubMed
-
- Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–1595. - PubMed
-
- Martineau L, Davis SC. Controlling methicillin resistant Staphyloccocus aureus and Pseudomonas aeruginosa wound infections with a novel biomaterial. Journal of Investigative Surgery. 2007;20(4):217–227. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

