Neonatal hyperoxia stimulates the expansion of alveolar epithelial type II cells
- PMID: 24188066
- PMCID: PMC4068921
- DOI: 10.1165/rcmb.2013-0207OC
Neonatal hyperoxia stimulates the expansion of alveolar epithelial type II cells
Abstract
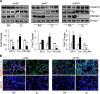
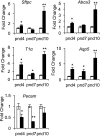
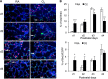
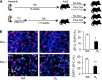
Supplemental oxygen used to treat infants born prematurely disrupts angiogenesis and is a risk factor for persistent pulmonary disease later in life. Although it is unclear how neonatal oxygen affects development of the respiratory epithelium, alveolar simplification and depletion of type II cells has been observed in adult mice exposed to hyperoxia between postnatal Days 0 and 4. Because hyperoxia inhibits cell proliferation, we hypothesized that it depleted the adult lung of type II cells by inhibiting their proliferation at birth. Newborn mice were exposed to room air (RA) or hyperoxia, and the oxygen-exposed mice were recovered in RA. Hyperoxia stimulated mRNA expressed by type II (Sftpc, Abca3) and type I (T1α, Aquaporin 5) cells and inhibited Pecam expressed by endothelial cells. 5-Bromo-2'-deoxyuridine labeling and fate mapping with enhanced green fluorescence protein controlled statically by the Sftpc promoter or conditionally by the Scgb1a1 promoter revealed increased Sftpc and Abca3 mRNA seen on Day 4 reflected an increase in expansion of type II cells shortly after birth. When mice were returned to RA, this expanded population of type II cells was slowly depleted until few were detected by 8 weeks. These findings reveal that hyperoxia stimulates alveolar epithelial cell expansion when it disrupts angiogenesis. The loss of type II cells during recovery in RA may contribute to persistent pulmonary diseases such as those reported in children born preterm who were exposed to supplemental oxygen.
Figures



Similar articles
-
Effect of recombinant IL-10 on cultured fetal rat alveolar type II cells exposed to 65%-hyperoxia.Respir Res. 2011 May 24;12(1):68. doi: 10.1186/1465-9921-12-68. Respir Res. 2011. PMID: 21609457 Free PMC article.
-
Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development.Am J Physiol Lung Cell Mol Physiol. 2006 Nov;291(5):L1101-11. doi: 10.1152/ajplung.00126.2006. Epub 2006 Jul 21. Am J Physiol Lung Cell Mol Physiol. 2006. PMID: 16861382
-
Dynamic Regulation of GH-IGF1 Signaling in Injury and Recovery in Hyperoxia-Induced Neonatal Lung Injury.Cells. 2021 Oct 29;10(11):2947. doi: 10.3390/cells10112947. Cells. 2021. PMID: 34831169 Free PMC article.
-
In vivo exposure to hyperoxia induces DNA damage in a population of alveolar type II epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2004 May;286(5):L1045-54. doi: 10.1152/ajplung.00376.2003. Epub 2004 Jan 16. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 14729512
-
Hyperoxia impairs postnatal alveolar epithelial development via NADPH oxidase in newborn mice.Am J Physiol Lung Cell Mol Physiol. 2009 Jul;297(1):L134-42. doi: 10.1152/ajplung.00112.2009. Epub 2009 May 1. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19411313 Free PMC article.
Cited by
-
Autophagy, TERT, and mitochondrial dysfunction in hyperoxia.Am J Physiol Heart Circ Physiol. 2021 Nov 1;321(5):H985-H1003. doi: 10.1152/ajpheart.00166.2021. Epub 2021 Sep 24. Am J Physiol Heart Circ Physiol. 2021. PMID: 34559580 Free PMC article.
-
Perinatal Hyperoxia and Developmental Consequences on the Lung-Brain Axis.Oxid Med Cell Longev. 2022 Feb 24;2022:5784146. doi: 10.1155/2022/5784146. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35251477 Free PMC article. Review.
-
Macrophage-derived IL-6 trans-signalling as a novel target in the pathogenesis of bronchopulmonary dysplasia.Eur Respir J. 2022 Feb 17;59(2):2002248. doi: 10.1183/13993003.02248-2020. Print 2022 Feb. Eur Respir J. 2022. PMID: 34446466 Free PMC article.
-
Perinatal origins of bronchopulmonary dysplasia-deciphering normal and impaired lung development cell by cell.Mol Cell Pediatr. 2023 Apr 18;10(1):4. doi: 10.1186/s40348-023-00158-2. Mol Cell Pediatr. 2023. PMID: 37072570 Free PMC article. Review.
-
Cerebral Oxygen Changes in Neonates During Immediate Transition After Birth and Early Life: An Observational Study.Drug Des Devel Ther. 2020 Nov 2;14:4703-4715. doi: 10.2147/DDDT.S266726. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33173280 Free PMC article.
References
-
- Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. - PubMed
-
- Coalson JJ, Winter VT, Gerstmann DR, Idell S, King RJ, Delemos RA. Pathophysiologic, morphometric, and biochemical studies of the premature baboon with bronchopulmonary dysplasia. Am Rev Respir Dis. 1992;145:872–881. - PubMed
-
- Maniscalco WM, Watkins RH, O’Reilly MA, Shea CP. Increased epithelial cell proliferation in very premature baboons with chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2002;283:L991–L1001. - PubMed
-
- Warner BB, Stuart LA, Papes RA, Wispé JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–L117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL097141/HL/NHLBI NIH HHS/United States
- R01 HL067392/HL/NHLBI NIH HHS/United States
- ES01247/ES/NIEHS NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- HL091968/HL/NHLBI NIH HHS/United States
- ES07026/ES/NIEHS NIH HHS/United States
- R01 ES007026/ES/NIEHS NIH HHS/United States
- R01 HL091968/HL/NHLBI NIH HHS/United States
- HL067392/HL/NHLBI NIH HHS/United States
- T32 ES007026/ES/NIEHS NIH HHS/United States
- R01 ES017250/ES/NIEHS NIH HHS/United States
- R01 HL097141/HL/NHLBI NIH HHS/United States
- HL66988/HL/NHLBI NIH HHS/United States
- T32 HL066988/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

