Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012
- PMID: 24188614
- PMCID: PMC3837674
- DOI: 10.3201/eid1911.130177
Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012
Abstract
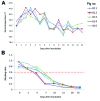
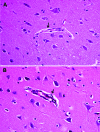
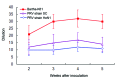
The widely used pseudorabies virus (PRV) Bartha-K61 vaccine has played a key role in the eradication of PRV. Since late 2011, however, a disease characterized by neurologic symptoms and a high number of deaths among newborn piglets has occurred among Bartha-K61-vaccinated pigs on many farms in China. Clinical samples from pigs on 15 farms in 6 provinces were examined. The PRV gE gene was detectable by PCR in all samples, and sequence analysis of the gE gene showed that all isolates belonged to a relatively independent cluster and contained 2 amino acid insertions. A PRV (named HeN1) was isolated and caused transitional fever in pigs. In protection assays, Bartha-K61 vaccine provided 100% protection against lethal challenge with SC (a classical PRV) but only 50% protection against 4 challenges with strain HeN1. The findings suggest that Bartha-K61 vaccine does not provide effective protection against PRV HeN1 infection.
Keywords: Bartha-K61 vaccine strain; China; immune evasion; pigs; pseudorabies virus; virulent; virus variant; viruses.
Figures


References
-
- Ketusing N, Reeves A, Portacci K, Yano T, Olea-Popelka F, Keefe T, et al. Evaluation of strategies for the eradication of pseudorabies virus (Aujeszky's disease) in commercial swine farms in Chiang-Mai and Lampoon Provinces, Thailand, using a simulation disease spread model. Transbound Emerg Dis. Epub 2012 Oct 3. - PubMed
-
- Yuan QZ, Li ZR, Nan X, Wu YX, Li YX. Isolation and identification of pseudorabies virus [in Chinese]. Chin J Prev Vet Med. 1987;3:10–1.
-
- Chen HX, Fang LR, He QG, Jin ML, Suo XF, Wu MZ. Study on the isolation and identification of the Ea strain of pseudorabies virus. Acta Veterinaria et Zootechnica Sinica. 1998;29:97–104.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
