Design and fabrication of human skin by three-dimensional bioprinting
- PMID: 24188635
- PMCID: PMC4024844
- DOI: 10.1089/ten.TEC.2013.0335
Design and fabrication of human skin by three-dimensional bioprinting
Abstract
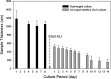
Three-dimensional (3D) bioprinting, a flexible automated on-demand platform for the free-form fabrication of complex living architectures, is a novel approach for the design and engineering of human organs and tissues. Here, we demonstrate the potential of 3D bioprinting for tissue engineering using human skin as a prototypical example. Keratinocytes and fibroblasts were used as constituent cells to represent the epidermis and dermis, and collagen was used to represent the dermal matrix of the skin. Preliminary studies were conducted to optimize printing parameters for maximum cell viability as well as for the optimization of cell densities in the epidermis and dermis to mimic physiologically relevant attributes of human skin. Printed 3D constructs were cultured in submerged media conditions followed by exposure of the epidermal layer to the air-liquid interface to promote maturation and stratification. Histology and immunofluorescence characterization demonstrated that 3D printed skin tissue was morphologically and biologically representative of in vivo human skin tissue. In comparison with traditional methods for skin engineering, 3D bioprinting offers several advantages in terms of shape- and form retention, flexibility, reproducibility, and high culture throughput. It has a broad range of applications in transdermal and topical formulation discovery, dermal toxicity studies, and in designing autologous grafts for wound healing. The proof-of-concept studies presented here can be further extended for enhancing the complexity of the skin model via the incorporation of secondary and adnexal structures or the inclusion of diseased cells to serve as a model for studying the pathophysiology of skin diseases.
Figures







References
-
- Bos J.D., and Meinardi M.H.The 500 dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol 9, 165, 2000 - PubMed
-
- Bouwstra J.A.The skin, a well organized membrane. Colloids Surf A 123, 403, 1997
-
- Karande P., and Mitragotri S.Transcutaneous immunization: an overview of advantages, disease targets, vaccines, and delivery technologies. In: Prausnitz J.M., Doherty M.F., and Segalman M.A., eds. Ann Rev Chem Biomol Eng 1, 175, 2010 - PubMed
-
- Adams D.C., and Ramsey M.L.Grafts in dermatologic surgery: review and update on full- and split-thickness skin grafts, free cartilage grafts, and composite grafts. Dermatol Surg 31, 1055, 2005 - PubMed
-
- Hierner R., Degreef H., Vranckx J.J., Garmyn M., Massage P., and van Brussel M.Skin grafting and wound healing - the “dermato-plastic team approach”. Clin Dermatol 23, 343, 2005 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

