Prostaglandins are essential for cervical ripening in LPS-mediated preterm birth but not term or antiprogestin-driven preterm ripening
- PMID: 24189143
- PMCID: PMC3868800
- DOI: 10.1210/en.2013-1304
Prostaglandins are essential for cervical ripening in LPS-mediated preterm birth but not term or antiprogestin-driven preterm ripening
Abstract
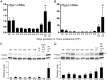
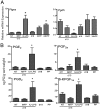
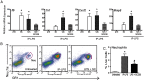
Globally, an estimated 13 million preterm babies are born each year. These babies are at increased risk of infant mortality and life-long health complications. Interventions to prevent preterm birth (PTB) require an understanding of processes driving parturition. Prostaglandins (PGs) have diverse functions in parturition, including regulation of uterine contractility and tissue remodeling. Our studies on cervical remodeling in mice suggest that although local synthesis of PGs are not increased in term ripening, transcripts encoding PG-endoperoxide synthase 2 (Ptgs2) are induced in lipopolysaccharide (LPS)-mediated premature ripening. This study provides evidence for two distinct pathways of cervical ripening: one dependent on PGs derived from paracrine or endocrine sources and the other independent of PG actions. Cervical PG levels are increased in LPS-treated mice, a model of infection-mediated PTB, consistent with increases in PG synthesizing enzymes and reduction in PG-metabolizing enzymes. Administration of SC-236, a PTGS2 inhibitor, along with LPS attenuated cervical softening, consistent with the essential role of PGs in LPS-induced ripening. In contrast, during term and preterm ripening mediated by the antiprogestin, mifepristone, cervical PG levels, and expression of PG synthetic and catabolic enzymes did not change in a manner that supports a role for PGs. These findings in mice, supported by correlative studies in women, suggest PGs do not regulate all aspects of the parturition process. Additionally, it suggests a need to refocus current strategies toward developing therapies for the prevention of PTB that target early, pathway-specific processes rather than focusing on common late end point mediators of PTB.
Figures




References
-
- Li R, Lam T-W, Kristiansen K, et al. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics (Oxford, England). 2009;25:1966–1967 - PubMed
-
- Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215 - PubMed
-
- Romero R, Munoz H, Gomez R, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids. 1996;54:187–191 - PubMed
-
- MacDonald PC, Casey ML. The accumulation of prostaglandins (PG) in amniotic fluid is an aftereffect of labor and not indicative of a role for PGE2 or PGF2 α in the initiation of human parturition. J Clin Endocrinol Metab. 1993;76:1332–1339 - PubMed
-
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong Cy. Labor Induction. In: Williams Obstetrics. 23rd ed New York: McGraw-Hill; 2010:chap 22
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

