Colitogenic effector T cells: roles of gut-homing integrin, gut antigen specificity and γδ T cells
- PMID: 24189163
- PMCID: PMC3947309
- DOI: 10.1038/icb.2013.70
Colitogenic effector T cells: roles of gut-homing integrin, gut antigen specificity and γδ T cells
Abstract
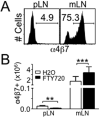
Disturbance of T-cell homeostasis could lead to intestinal inflammation. Naive CD4 T cells undergoing spontaneous proliferation, a robust proliferative response that occurs under severe lymphopenic conditions, differentiate into effector cells producing Th1- and/or Th17-type cytokines and induce a chronic inflammation in the intestine that resembles human inflammatory bowel disease. In this study, we investigated the key properties of CD4 T cells necessary to induce experimental colitis. α4β7 upregulation was primarily induced by mesenteric lymph node (mLN) resident CD11b(+) dendritic cell subsets via transforming growth factor beta (TGFβ)/retinoic acid-dependent mechanism. Interestingly, α4β7 expression was essential but not sufficient to induce inflammation. In addition to gut-homing specificity, expression of gut Ag specificity was also crucial. T-cell acquisition of the specificity was dramatically enhanced by the presence of γδ T cells, a population previously shown to exacerbate T-cell-mediated colitis. Importantly, interleukin (IL)-23-mediated γδ T cell stimulation was necessary to enhance colitogenicity but not gut antigen reactivity of proliferating CD4 T cells. These findings demonstrate that T-cell colitogenicity is achieved through multiple processes, offering a therapeutic rationale by intervening these pathways.
Figures




References
-
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. - PubMed
-
- Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang HQ, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–63. - PubMed
-
- Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev. 2007;215:226–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

