Effects of soybean agglutinin on mechanical barrier function and tight junction protein expression in intestinal epithelial cells from piglets
- PMID: 24189218
- PMCID: PMC3856029
- DOI: 10.3390/ijms141121689
Effects of soybean agglutinin on mechanical barrier function and tight junction protein expression in intestinal epithelial cells from piglets
Abstract
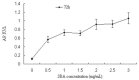
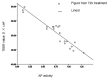
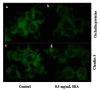
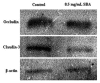
In this study, we sought to investigate the role of soybean agglutinin (SBA) in mediating membrane permeability and the mechanical barrier function of intestinal epithelial cells. The IPEC-J2 cells were cultured and treated with 0, 0.5, 1.0, 1.5, 2.0, 2.5, or 3.0 mg/mL SBA. Transepithelial electrical resistance (TEER) and alkaline phosphatase (AP) activity were measured to evaluate membrane permeability. The results showed a significant decrease in TEER values (p < 0.05) in a time- and dose-dependent manner, and a pronounced increase in AP activity (p < 0.05). Cell growth and cell morphology were used to evaluate the cell viability. A significant cell growth inhibition (p < 0.05) and alteration of morphology were observed when the concentration of SBA was increased. The results of western blotting showed that the expression levels of occludin and claudin-3 were decreased by 31% and 64% compared to those of the control, respectively (p < 0.05). In addition, immunofluorescence labeling indicated an obvious decrease in staining of these targets and changes in their localizations. In conclusion, SBA increased the membrane permeability, inhibited the cell viability and reduced the levels of tight junction proteins (occludin and claudin-3), leading to a decrease in mechanical barrier function in intestinal epithelial cells.
Figures



Similar articles
-
Mitigative effects of Eleutheroside E against the mechanical barrier dysfunction induced by soybean agglutinin in IPEC-J2 cell line.J Anim Physiol Anim Nutr (Berl). 2022 May;106(3):664-670. doi: 10.1111/jpn.13677. Epub 2022 Jan 10. J Anim Physiol Anim Nutr (Berl). 2022. PMID: 35014099
-
Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets.Int J Mol Sci. 2011;12(12):8502-12. doi: 10.3390/ijms12128502. Epub 2011 Nov 29. Int J Mol Sci. 2011. PMID: 22272087 Free PMC article.
-
N-Acetyl-d-galactosamine prevents soya bean agglutinin-induced intestinal barrier dysfunction in intestinal porcine epithelial cells.J Anim Physiol Anim Nutr (Berl). 2019 Jul;103(4):1198-1206. doi: 10.1111/jpn.13091. Epub 2019 Apr 1. J Anim Physiol Anim Nutr (Berl). 2019. PMID: 30934149
-
Eleutheroside B increase tight junction proteins and anti-inflammatory cytokines expression in intestinal porcine jejunum epithelial cells (IPEC-J2).J Anim Physiol Anim Nutr (Berl). 2019 Jul;103(4):1174-1184. doi: 10.1111/jpn.13087. Epub 2019 Apr 16. J Anim Physiol Anim Nutr (Berl). 2019. PMID: 30990939
-
β-Conglycinin reduces the tight junction occludin and ZO-1 expression in IPEC-J2.Int J Mol Sci. 2014 Jan 27;15(2):1915-26. doi: 10.3390/ijms15021915. Int J Mol Sci. 2014. PMID: 24473141 Free PMC article.
Cited by
-
Research on the Mechanism of HRP Relieving IPEC-J2 Cells Immunological Stress Based on Transcriptome Sequencing Analysis.Front Nutr. 2022 Jul 15;9:944390. doi: 10.3389/fnut.2022.944390. eCollection 2022. Front Nutr. 2022. PMID: 35911118 Free PMC article.
-
The Influences of Soybean Agglutinin and Functional Oligosaccharides on the Intestinal Tract of Monogastric Animals.Int J Mol Sci. 2018 Feb 12;19(2):554. doi: 10.3390/ijms19020554. Int J Mol Sci. 2018. PMID: 29439523 Free PMC article. Review.
-
Characterization of anti-soybean agglutinin (SBA) IgY antibodies: a new strategy for neutralization of the detrimental biological activity of SBA.Front Vet Sci. 2024 Apr 12;11:1382510. doi: 10.3389/fvets.2024.1382510. eCollection 2024. Front Vet Sci. 2024. PMID: 38681857 Free PMC article.
-
LncRNA446 Regulates Tight Junctions by Inhibiting the Ubiquitinated Degradation of Alix after Porcine Epidemic Diarrhea Virus Infection.J Virol. 2023 Mar 30;97(3):e0188422. doi: 10.1128/jvi.01884-22. Epub 2023 Feb 15. J Virol. 2023. PMID: 36790206 Free PMC article.
-
Dietary emodin alleviates lipopolysaccharide-induced intestinal mucosal barrier injury by regulating gut microbiota in piglets.Anim Nutr. 2023 May 16;14:152-162. doi: 10.1016/j.aninu.2023.05.004. eCollection 2023 Sep. Anim Nutr. 2023. PMID: 37455790 Free PMC article.
References
-
- Irish G.G., Maenz D.D., Classen H.L. A new assay for functional lectins: the brush border lectin agglutinin assay (BBLAA) Anim. Feed Sci. Technol. 1999;76:321–333.
-
- Dorland L., van-Halbcek H., Vliegenthart J.F.G., Lis H., Sharon N. Primary structure of the carbohydrate chain of soybean agglutinin. J. Biol. Chem. 1981;256:7708–7711. - PubMed
-
- King T.P., Begbie R., Cadenhead A. Nutritional toxicity of raw kidney beans in pigs. Immunocytochemical and cytopathological studies on the gut and the pancreas. J. Sci. Food Agric. 1983;34:1404–1412. - PubMed
-
- Quaroni A., Hochman J. Development of intestinal cell culture models for drug transport and metabolism studies. Adv. Drug Deliv. Rev. 1996;22:3–52.
-
- Pinton P., Nougayrède J.P., Del Rio J.C., Moreno C., Marin D.E., Ferrier L., Bracarense A.P., Kolf-Clauw M., Oswald I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009;237:41–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

