A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid
- PMID: 24189246
- PMCID: PMC3910734
- DOI: 10.1128/AAC.01558-13
A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid
Abstract
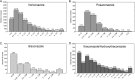
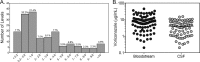
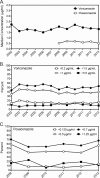
Interest in antifungal therapeutic-drug monitoring has increased due to studies demonstrating associations between concentrations and outcomes. We reviewed the antifungal drug concentration database at our institution to gain a better understanding of achievable triazole drug levels. Antifungal concentrations were measured by high-performance liquid chromatography (HPLC), ultraperformance liquid chromatography and single-quadrupole mass spectrometry (UPLC/MS), or a bioassay. For this study, only confirmed human bloodstream (serum or plasma) and cerebral spinal fluid (CSF) concentrations of voriconazole, posaconazole, and itraconazole were analyzed. The largest numbers of bloodstream and CSF samples were found for voriconazole (14,370 and 173, respectively). Voriconazole bloodstream concentrations within the range of 1 to 5.5 μg/ml represented 50.6% of samples. Levels below the lower limit of quantification (0.2 μg/ml) were observed in 14.6% of samples, and 10.4% of samples had levels of ≥5.5 μg/ml. CSF voriconazole levels ranged from undetectable to 15.3 μg/ml and were <0.2 μg/ml in 11% of samples. Posaconazole bloodstream concentrations were ≥0.7 and ≥1.25 μg/ml in 41.6% and 18.9% of samples, respectively. Posaconazole was detected in only 4 of 22 CSF samples (undetectable to 0.56 μg/ml). Itraconazole levels, as measured by UPLC/MS, were ≥0.5 μg/ml in 43.3% and were undetectable in 33.9% of bloodstream samples. In contrast, when measured by a bioassay, itraconazole/hydroxyitraconazole bloodstream concentrations were ≥1.0 μg/ml in 72.9% of samples and were undetectable in 18% of samples. These results indicate that there is marked variability in bloodstream concentrations achieved with these three azoles. In addition, many levels within the bloodstream for each azole and for voriconazole and posaconazole in the CSF were undetectable or below thresholds associated with efficacy.
Figures
Similar articles
-
Multiplex ultra-performance liquid chromatography-tandem mass spectrometry method for simultaneous quantification in human plasma of fluconazole, itraconazole, hydroxyitraconazole, posaconazole, voriconazole, voriconazole-N-oxide, anidulafungin, and caspofungin.Antimicrob Agents Chemother. 2010 Dec;54(12):5303-15. doi: 10.1128/AAC.00404-10. Epub 2010 Sep 20. Antimicrob Agents Chemother. 2010. PMID: 20855739 Free PMC article.
-
Validation of a fast and reliable liquid chromatography-tandem mass spectrometry (LC-MS/MS) with atmospheric pressure chemical ionization method for simultaneous quantitation of voriconazole, itraconazole and its active metabolite hydroxyitraconazole in human plasma.Clin Chem Lab Med. 2013 Feb;51(2):339-46. doi: 10.1515/cclm-2012-0364. Clin Chem Lab Med. 2013. PMID: 23095205
-
Development, validation, and routine application of a high-performance liquid chromatography method coupled with a single mass detector for quantification of itraconazole, voriconazole, and posaconazole in human plasma.Antimicrob Agents Chemother. 2010 Aug;54(8):3408-13. doi: 10.1128/AAC.01807-09. Epub 2010 Jun 7. Antimicrob Agents Chemother. 2010. PMID: 20530230 Free PMC article.
-
Triazole antifungal agents in invasive fungal infections: a comparative review.Drugs. 2011 Dec 24;71(18):2405-19. doi: 10.2165/11596540-000000000-00000. Drugs. 2011. PMID: 22141384 Review.
-
Antifungal Therapy with Azoles Induced the Syndrome of Acquired Apparent Mineralocorticoid Excess: a Literature and Database Analysis.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0166821. doi: 10.1128/AAC.01668-21. Epub 2021 Oct 18. Antimicrob Agents Chemother. 2022. PMID: 34662186 Free PMC article.
Cited by
-
Impact of Triazole Therapeutic Drug Monitoring Availability and Timing.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e01245-19. doi: 10.1128/AAC.01245-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31332058 Free PMC article.
-
Modeling and Simulation as a Tool to Assess Voriconazole Exposure in the Central Nervous System.Pharmaceutics. 2023 Jun 21;15(7):1781. doi: 10.3390/pharmaceutics15071781. Pharmaceutics. 2023. PMID: 37513968 Free PMC article.
-
The Novel Fungal Cyp51 Inhibitor VT-1598 Is Efficacious in Experimental Models of Central Nervous System Coccidioidomycosis Caused by Coccidioides posadasii and Coccidioides immitis.Antimicrob Agents Chemother. 2018 Mar 27;62(4):e02258-17. doi: 10.1128/AAC.02258-17. Print 2018 Apr. Antimicrob Agents Chemother. 2018. PMID: 29437615 Free PMC article.
-
MSG-15: Super-Bioavailability Itraconazole Versus Conventional Itraconazole in the Treatment of Endemic Mycoses-A Multicenter, Open-Label, Randomized Comparative Trial.Open Forum Infect Dis. 2024 Jan 29;11(3):ofae010. doi: 10.1093/ofid/ofae010. eCollection 2024 Mar. Open Forum Infect Dis. 2024. PMID: 38440302 Free PMC article.
-
Antifungal Triazole Posaconazole Targets an Early Stage of the Parechovirus A3 Life Cycle.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e02372-19. doi: 10.1128/AAC.02372-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31818821 Free PMC article.
References
-
- Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, Song KH, Choe PG, Kim NJ, Jang IJ, Oh MD, Yu KS. 2012. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin. Infect. Dis. 55:1080–1087. 10.1093/cid/cis599 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

