CD133 is a positive marker for a distinct class of primitive human cord blood-derived CD34-negative hematopoietic stem cells
- PMID: 24189293
- PMCID: PMC4051213
- DOI: 10.1038/leu.2013.326
CD133 is a positive marker for a distinct class of primitive human cord blood-derived CD34-negative hematopoietic stem cells
Abstract
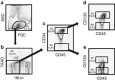
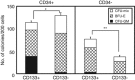
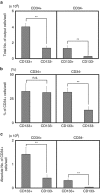
The identification of human CD34-negative (CD34(-)) hematopoietic stem cells (HSCs) provides a new concept for the hierarchy in the human HSC compartment. Previous studies demonstrated that CD34(-) severe combined immunodeficiency (SCID)-repopulating cells (SRCs) are a distinct class of primitive HSCs in comparison to the well-characterized CD34(+)CD38(-) SRCs. However, the purification level of rare CD34(-) SRCs in 18 lineage marker-negative (Lin(-)) CD34(-) cells (1/1000) is still very low compared with that of CD34(+)CD38(-) SRCs (1/40). As in the mouse, it will be necessary to identify useful positive markers for a high degree of purification of rare human CD34(-) SRCs. Using 18Lin(-)CD34(-) cells, we analyzed the expression of candidate positive markers by flow cytometric analysis. We finally identified CD133 as a reliable positive marker of human CB-derived CD34(-) SRCs and succeeded in highly purifying primitive human CD34(-) HSCs. The limiting dilution analysis demonstrated that the incidence of CD34(-) SRCs in 18Lin(-)CD34(-)CD133(+) cells was 1/142, which is the highest level of purification of these unique CD34(-) HSCs to date. Furthermore, CD133 expression clearly segregated the SRC activities of 18Lin(-)CD34(-) cells, as well as 18Lin(-)CD34(+) cells, in their positive fractions, indicating its functional significance as a common cell surface maker to isolate effectively both CD34(+) and CD34(-) SRCs.
Figures



References
-
- Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. - PubMed
-
- Ratajczak MZ. Phenotypic and functional characterization of hematopoietic stem cells. Curr Opin Hematol. 2008;15:293–300. - PubMed
-
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. - PubMed
-
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

