Targeting PARP-1 allosteric regulation offers therapeutic potential against cancer
- PMID: 24189460
- PMCID: PMC3903668
- DOI: 10.1158/0008-5472.CAN-13-1701
Targeting PARP-1 allosteric regulation offers therapeutic potential against cancer
Abstract
PARP-1 is a nuclear protein that has important roles in maintenance of genomic integrity. During genotoxic stress, PARP-1 recruits to sites of DNA damage where PARP-1 domain architecture initiates catalytic activation and subsequent poly(ADP-ribose)-dependent DNA repair. PARP-1 inhibition is a promising new way to selectively target cancers harboring DNA repair deficiencies. However, current inhibitors target other PARPs, raising important questions about long-term off-target effects. Here, we propose a new strategy that targets PARP-1 allosteric regulation as a selective way of inhibiting PARP-1. We found that disruption of PARP-1 domain-domain contacts through mutagenesis held no cellular consequences on recruitment to DNA damage or a model system of transcriptional regulation, but prevented DNA-damage-dependent catalytic activation. Furthermore, PARP-1 mutant overexpression in a pancreatic cancer cell line (MIA PaCa-2) increased sensitivity to platinum-based anticancer agents. These results not only highlight the potential of a synergistic drug combination of allosteric PARP inhibitors with DNA-damaging agents in genomically unstable cancer cells (regardless of homologous recombination status), but also signify important applications of selective PARP-1 inhibition. Finally, the development of a high-throughput PARP-1 assay is described as a tool to promote discovery of novel PARP-1 selective inhibitors.
Conflict of interest statement
The authors declare no conflict of interest
Figures

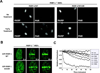
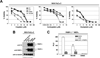

References
-
- Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci. 2008;13:3046–3082. - PubMed
-
- Langelier MF, Servent KM, Rogers EE, Pascal JM. A third zinc-binding domain of human poly(ADP-ribose) polymerase-1 coordinates DNA-dependent enzyme activation. J Biol Chem. 2008;283(7):4105–4114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

